- Biological Therapies
- Il 12/23 Inhibitor (Ustekinumab)
- Il-1 Antagonist
- B Cell Directed Biological Therapies
- Omalizumab
- Anti TB drugs
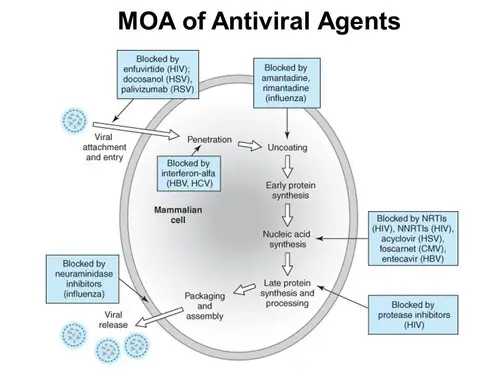
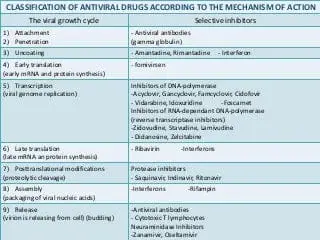
- Antivirals
- Methotrexate
- Dapsone
- Ciclosporin
- Topical steroids
- Systemic steroids
- Azathioprine
- Topical Vitamin D Analogues
- Potassium iodide
- Pentavalent Antimony Glucantime
- Thalidomide
- Cyclophosphomide
- Systemic retinoids
- Topical retinoid
- Secukinumab
- Apremilast
- JAK inhibitors
- Fumaric acid esters
- Antimalarials
- Colchicine
- TNF Alpha Inhibitors
- MMF
- IV Immunoglobulin
- Clofazamine
- Rituximab
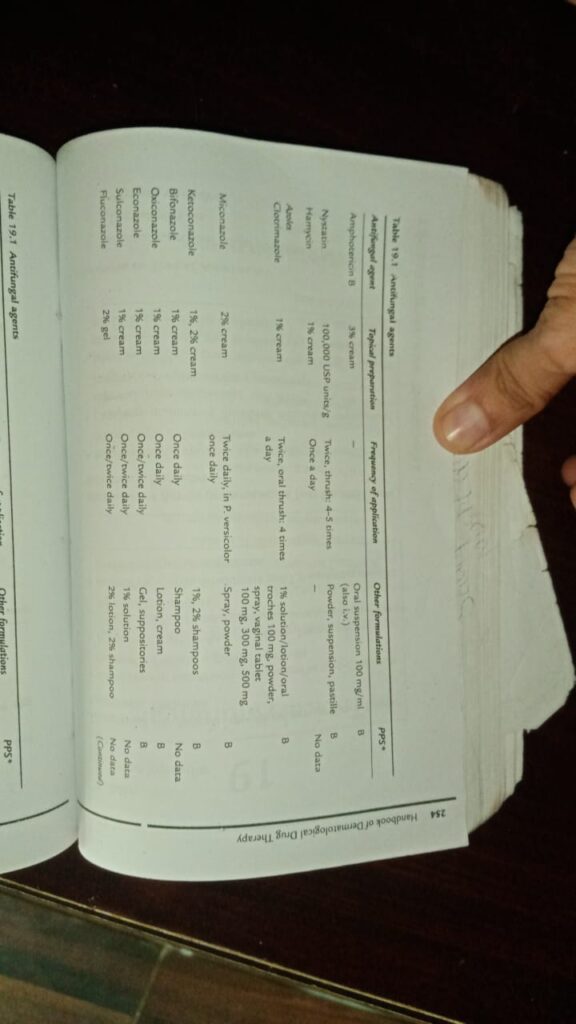
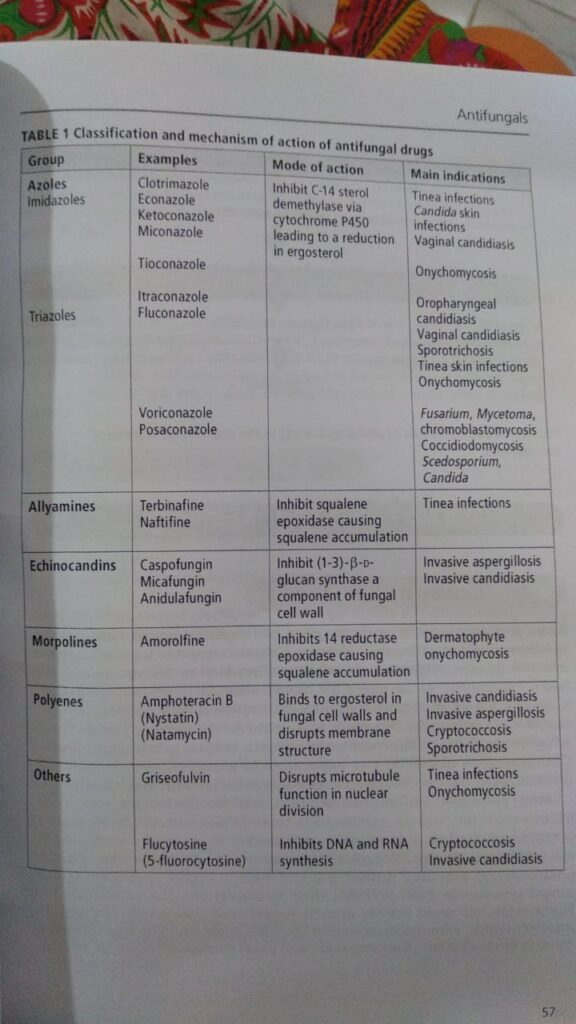
- Antifungals

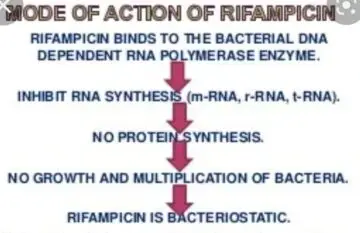





Rifampicin
Side effects
– Effects of bodily fluids
Tears, sweat and urine may become orange coloured by oral rifampicin. Contact lenses may be
permanently stained. This effect is usually mild and self-limiting.
Cutaneous adverse reactions
. flushing
. itching
Drug-induced urticaria
Erythema multiforme
Toxic epidermal necrolysis (rare).
Gastrointestinal effects
Rifampicin may cause loss of appetite, vomiting, abdominal pain and diarrhoea.
Liver disease
Hepatitis can be caused by rifampicin, particularly if rifampicin is given with isoniazid.
Blood disorders
Serious haematological disorders have been reported in patients taking rifampicin, including:
Thrombocytopenia (low platelet count), which may potentially result in bruising and bleeding
Low white blood cell count; very rarely, agranulocytosis (severely decreased white blood cell counts)
Disseminated intravascular coagulation (also very rare).
Musculoskeletal
Muscle weakness and myopathy are uncommon side effects of rifampicin.
Monitoring of rifampicin
Do blood count, renal function tests, and liver function tests.
If there are significant abnormalities, these should be repeated during treatment. Caution should be
taken when there is a pre-existing liver disease or liver function abnormalities.
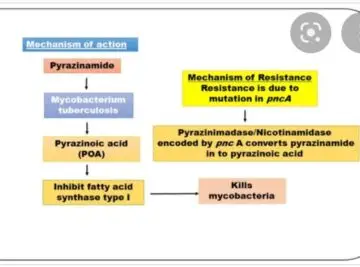
Pyrizinamide
Side effects
Loss of appetite
Nausea
Vomiting
Muscle fatigue
Joint pains : hyperuricemia
Monitoring :
Uric acid
LFts
——————————————————————————————-
What is XDR TB
Extensively drug-resistant TB (XDR TB) is a rare type of multidrug-resistant tuberculosis (MDR TB) that is resistant to isoniazid and rifampin, plus any fluoroquinolone and at least one of three injectable secondline drugs (i.e., amikacin, kanamycin, or capreomycin).
MANAGEMENT FOR PATIENTS WITH DOCUMENTED, OR ALMOST CERTAIN, XDR-TB
Use pyrazinamide and any other Group 1 agent that may be effective.
Use an injectable agent to which the strain is susceptible and consider an extended duration of use (12 months or possibly the whole treatment).
If resistant to all injectable agents it is recommended to use one the patient has never used before or consider designing the regimen without an injectable agent.
Use a higher-generation fluoroquinolone such as moxifloxacin or gatifloxacin.
Use all Group 4 agents that have not been used extensively in a previous regimen or any that are likely to be effective.
Add two or more Group 5 drugs (consider adding bedaquiline ).
Consider high-dose isoniazid treatment if low-level resistance or absence of the katG gene is documented.
Consider adjuvant surgery if there is localized disease.

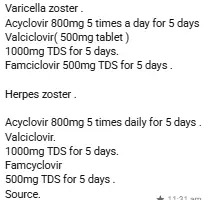


Antiviral drug interactions are a particular problem among immuno-compromised patients because these patients are often receiving multiple different drugs, i.e. antiretroviral drugs and drugs effective against herpesvirus. e.g
📍1 )Coadministration of zidovudine and ganciclovir should be avoided because of the high rate of haematological intolerance
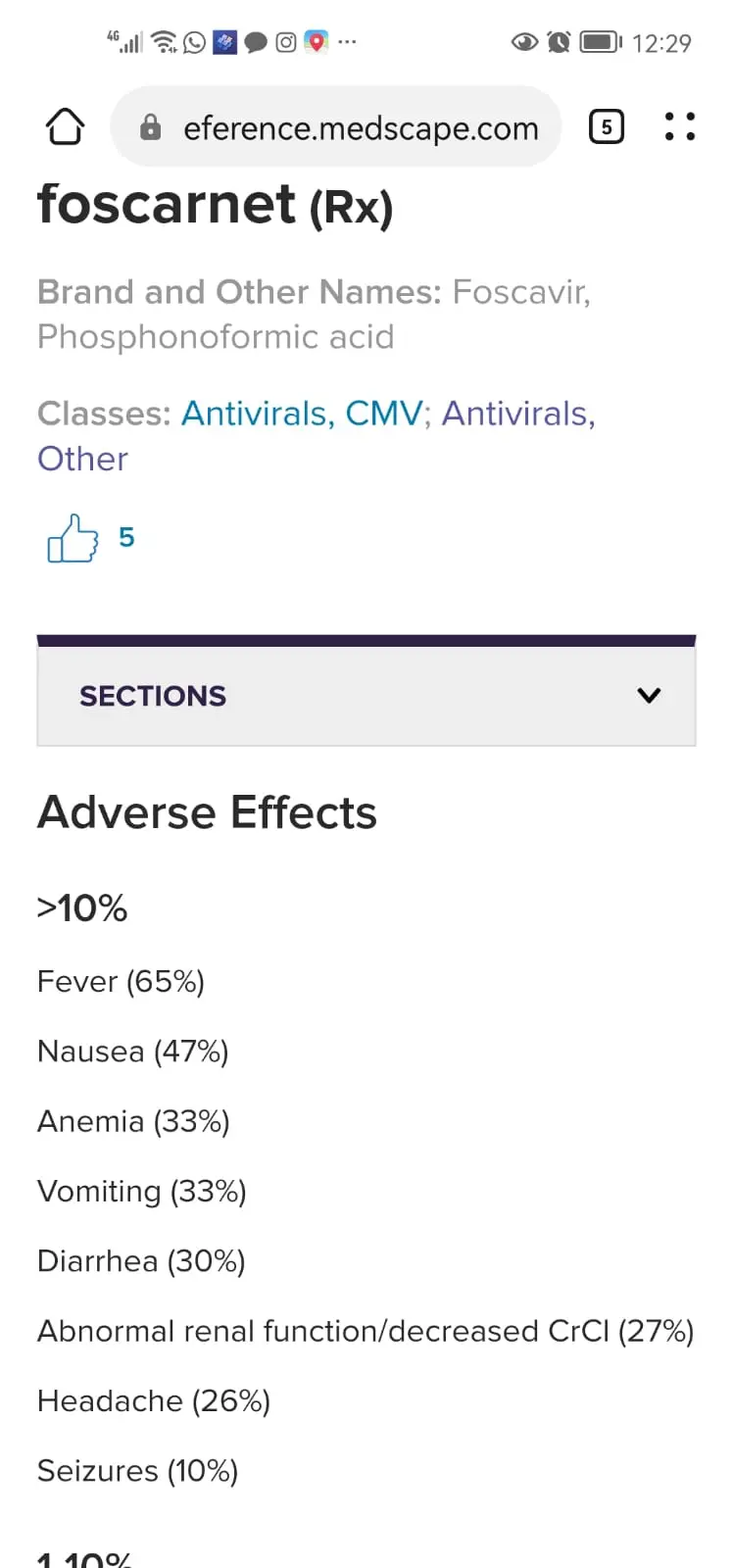
📍 2 ) , zidovudine and foscarnet
have synergistic effect and no pharmacokinetic interaction has been detected.
📍3) NSAIDS interact with antivirals .Thsed drugs that may cause kidney problems (including ibuprofen, naproxen).
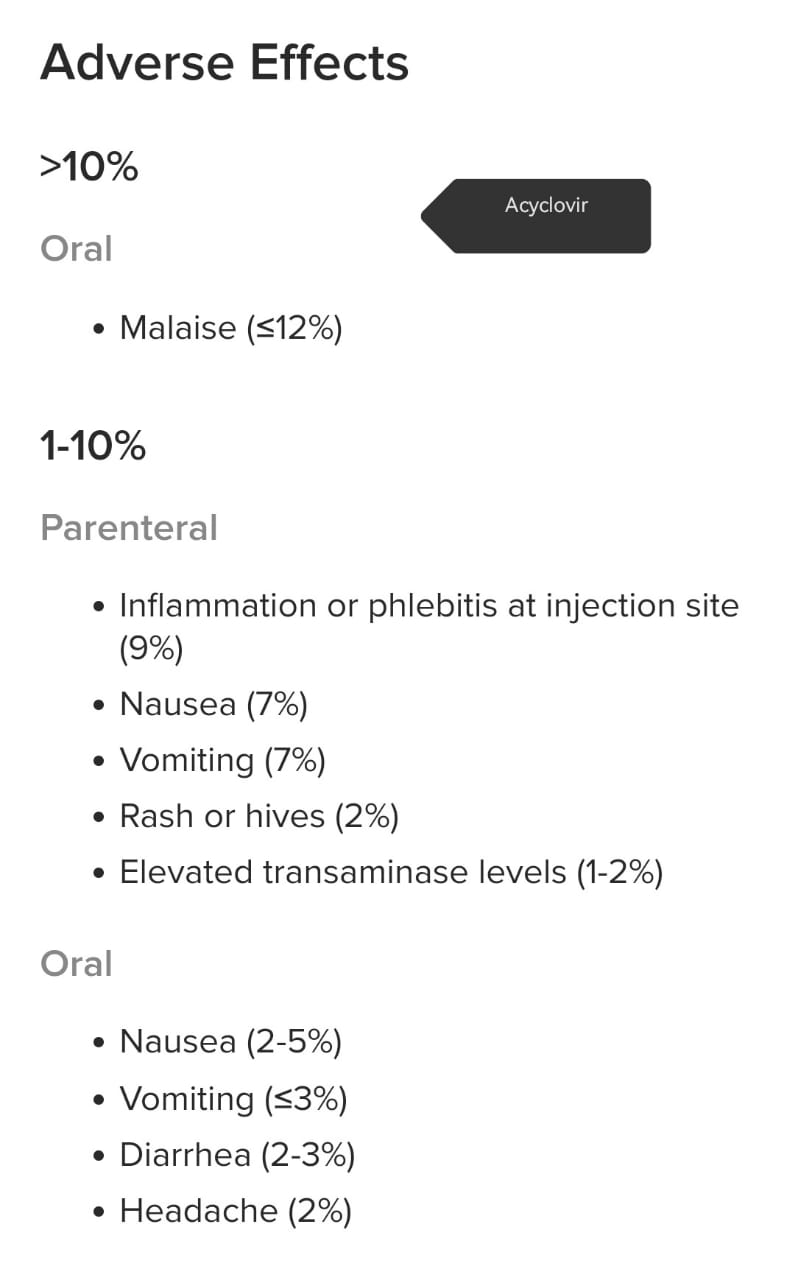
📍 4) Drug interactions of zovirax(acyclovir) includes:
*
- i) Phenytoin,
- ii) Valproic acid,
iii) Probenecid,
- iv) Theophylline,
- v) Cidofovir,
- vi) Amphotericin B or other drugs that reduce kidney function.

https://www.medicinenet.com ›
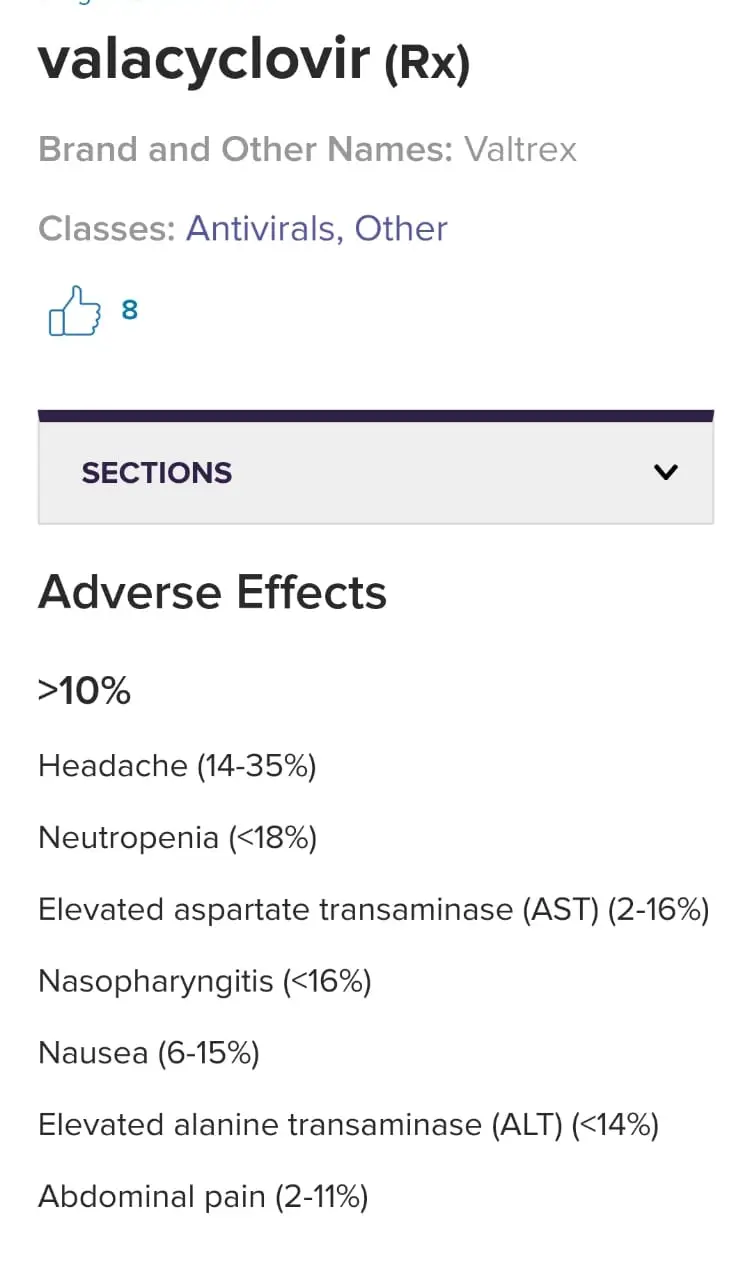
📍5) Valacyclovir has no known severe interactions with other drugs
📍 6 ) Acyclovir is very similar to valacyclovir. Do not use medications containing valacyclovir while using acyclovir.












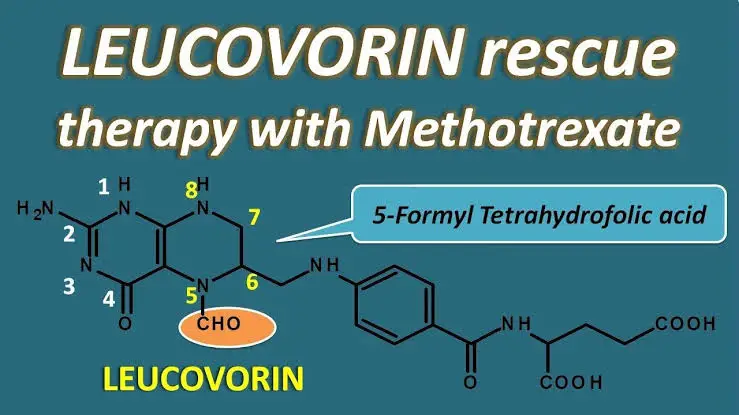






what are the side effects of mtx and how are they minimized?
🛑Systemic side effects of MTX 🛑
1. MYELOTOXICITY
( Anemia , leucopenia, thrombocytopenia ) all or any one of them —during 1st 2 months of starting Rx
but can occur at any time.
Risk factors for myelotoxicity= poor renal function, old age, NSAIDs, Hypoalbuminemia, daily intake,lack
of folate supplementation, drug interactions like asprin, sulfonamides
2. HEPATOTOXICITY
Acutely Increased transaminase levels , chronically Liver fibrosis ( with 2 to 10 yrs of Rx )
Risk factors for hepatotoxicity = Obesity, Alcoholism, previous hepatic dysfunction, diabetes,
hypoalbuminemia, concurrent administration of other hepatotoxic agents
Mech of metho related liver fibrosis=
Adosine A2a G-protein coupled receptors involved
3. GI
Nausea, vomiting, anorexia,diarrhea, stomatitis,
4. NEPHRO
Renal tubular damage
5. REPRODUCTIVE
Abortificent, teratogenic,mutagenic, irregular menstruation, impairs spermatogenesis and oogenesis
6. PULMONARY
Acute pneumonitis
Lung fibrosis
Cutaneous side effects of mtx:
Oral ulceration,stomatitis
Skin ulceration of lower legs
Recall reaction
Acral erythema,epidermal necrosis
Rapid ulceration of psoriatic plaques
🔴 How are side effects minimized🔴
Folate supplementation protects some protection from myelotoxicity
GIT side effects can be minimized by supplementation with folate or parenter admission or split dose or
by giving injection subcutaneously
Take with food
If possible, reduce the dose
Divide dose from once weekly into three 12-hourly doses
Take anti-emetic
Men and women both avoid conception during and 6 months after stopping methotrexate








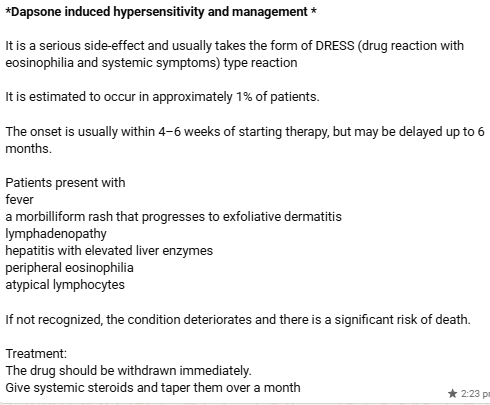
*Mechanism of action of dapsone
Antibacterial
Antiinflammatory
1) causes decrease bacterial synthesis of dihydrofolate by competing with paraaminobenzoic
acid for catalytic activity of dihydroterate synthetase
2 ) antiinflammatory action
* Inhibits neutrophil and eosinophil myeloperoxidase
* inhibit neutrophilic chemotaxis
. Stabilize the neutrophil lyzozymes
. inhibit neutrophilic lysosomal enzymes
Inhibit neutrophil recruitment
G6pD is rate limiting step in pentose phosphate pathway that produces NADPH WHICH
MAINTAINS SUPPLY OF reduced glutathione that prevents accumulation of free radicals
When G6PD level is defecient defence against oxidative stress is lost which causes damage to
RBc plasma membrane causes haemolysis and phagocytosis and oxidation of ferous ion into
feric ion
Indications of dapsone
1) leprosy as MDT
2) IMMUNOBULLOUS ( DH ,LINEAR IGA , BULLOUS SLE ‘IgA pemphigus ,BP ,
Pemphigus group
3) vasculitis (EED) granuloma faciale, uv , bechet
4) neutrophilic ( sweet ,pg, subcorneal pustular dermatosis )
5) acne vulgaris
6) LE
7) Panniculitis
8) Relapsing polychondritis







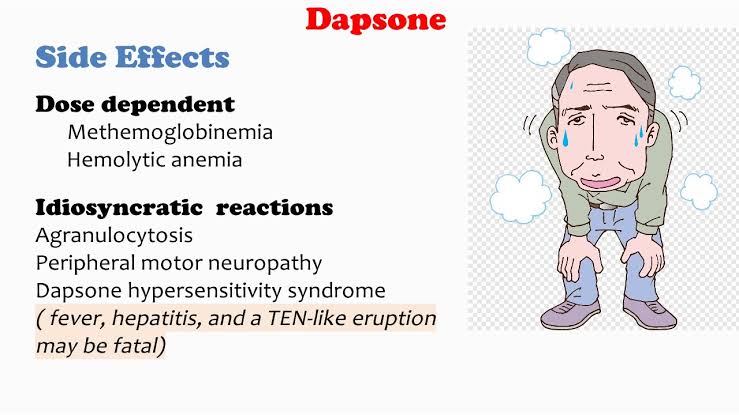



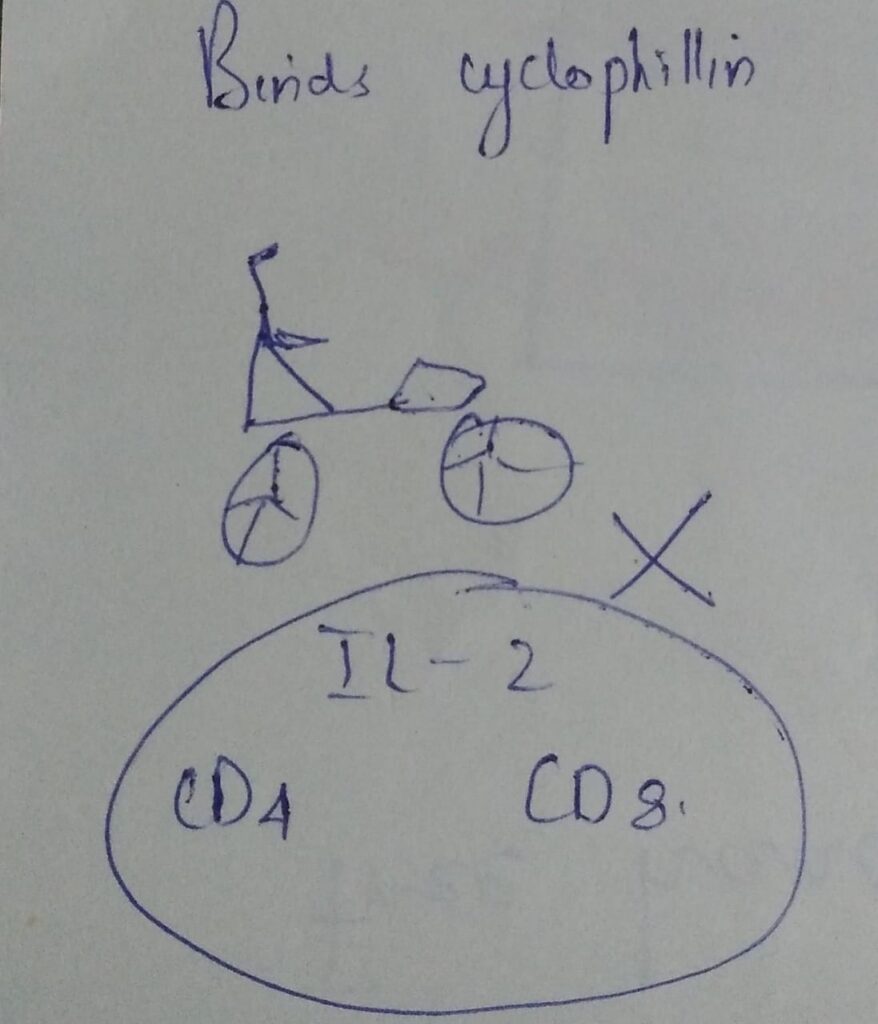
Dose related side effects of ciclosporin
most common side effects
- hypertension
- nephrotoxicity
- hyperlipidemia
- myalgia
- headache
less common:
- gingival hyperplasia
- fatigue
- GI disturbances
- tremors
- paresthesia of hands or feet
- metabolic abnormalities (hyperbilirubinemia, hypercalcemia, hypomagnesemia, hyperuricemia)
NEPHROTOXICITY :
ACUTE: occurs within weeks of treatment initiation and is dose related
there is vascular dysfuntion involving afferent arteriolar constriction leading to increased vascular resistance and decreased GFR
tubular dysfunction is characterized by decreased mg reabsorption, decreased uric acid secretion, decreased K+ and hydrogen ion secretion and distal tubular acidosis
chronic nephotoxicity: irreversible
risk factors: age over 50, preexisting HTN or renal failure or treatment with NSAIDS
dose-minimizing strategy : intermittent rather than continuous treatment
CHANGE IN S. CREATININE is SINGLE MOST important indicator of nephrotoxicity
HYPERLIPIDEMIA: triglycerides are raised, less commonly cholesterol within 2 weeks of treatment… they usually return to normal on withdrawl of therapy. Management: fibrates (statin-induced myopathy)
MALIGNANCY: increased risk of cancers of skin (esp SCC) and lymphoid system
Dose related side effects of ciclosporin
most common side effects
1. hypertension
2. nephrotoxicity
3. hyperlipidemia
4. myalgia
5. headache
less common:
1. gingival hyperplasia
2. fatigue
3. GI disturbances
4. tremors
5. paresthesia of hands or feet
6. metabolic abnormalities (hyperbilirubinemia, hypercalcemia, hypomagnesemia, hyperuricemia)
NEPHROTOXICITY :
ACUTE: occurs within weeks of treatment initiation and is dose related
there is vascular dysfuntion involving afferent arteriolar constriction leading to increased vascular
resistance and decreased GFR
tubular dysfunction is characterized by decreased mg reabsorption, decreased uric acid secretion,
decreased K+ and hydrogen ion secretion and distal tubular acidosis
chronic nephotoxicity: irreversible
risk factors: age over 50, preexisting HTN or renal failure or treatment with NSAIDS
dose-minimizing strategy : intermittent rather than continuous treatment
CHANGE IN S. CREATININE is SINGLE MOST important indicator of nephrotoxicity
HYPERLIPIDEMIA: triglycerides are raised, less commonly cholesterol within 2 weeks of treatment… they
usually return to normal on withdrawl of therapy. Management: fibrates (statin-induced myopathy)
MALIGNANCY: increased risk of cancers of skin (esp SCC) and lymphoid system


*The administered dose of ciclosporin* is based on
➕patient’s body weight
Avoid dosing per kg body wt in obese patients to avoid toxity
➕condition being treated usually at a dosage of 2–15 mg/kg per day in divided doses.
The dose can then be increased or decreased by 25–50 mg at the time of the next administration
After disease remission is achieved, usually after 6–8 weeks of treatment, the dose of ciclosporin can be
slowly reduced. Ciclosporin can eventually be given intermittently, such as twice weekly, as
maintenance therapy. This is often continued for a longer period, such as up to 1 year
🔷In patients with severe psoriasis, in which a rapid response is needed, an initial dose of 5 mg per kg
daily is usually a better option.
🔷Although the higher dose is associated with a faster and more efficacious response, it is also
associated with a higher rate of adverse reactions.
🔷Clinical improvement of the cutaneous lesions occurs after approximately 4 weeks, and maximum
response is seen after 8 to 16 weeks.
🔷If a satisfactory response is not achieved after 4 to 6 weeks of initial therapy with the lower dose (2.5
mg per kg daily), the dose can be increased gradually by 0.5 to 1.0 mg/kg/day at 2- to 4-week intervals,
to a maximum of 5 mg/kg/day, as long as, the laboratory parameters remain satisfactory.
🔷If response is still unsatisfactory after 3 months of treatment with the higher doses, then cyclosporine
should be discontinued.
*Long-Term Therapy*
Currently, long-term therapy of psoriasis (> 1 year) with cyclosporine is not a common approach and
should be prescribed only after other therapeutic options have been considered. This is because of
possible adverse effects, including ▪️renal toxicity
▪️arterial hypertension
▪️increased risk of developing lymphoproliferative disorders and other malignant tumors
▪️ Current guidelines limit the continuous use of cyclosporine in the United States to 1 year,whereas in
Europe the recommended limit is 2 years.
*Short-Term Therapy*
The use of intermittent short-term therapy is currently the most commonly recommended regimen of
cyclosporine for the treatment of psoriasis.Patients are treated until an adequate response is achieved,
which generally requires 8 to 16 weeks. Subsequently, cyclosporine is discontinued or slowly tapered by
1 mg per kg every week over 4 weeks
▪️A short course of cyclosporine can be used in severe flares of psoriasis because of its rapid onset of
action until a better long-term alternative treatment is instituted. This is particularly useful in the
treatment of erythrodermic or generalized pustular psoriasis where cyclosporine remains as the
treatment of choice despite the new biologic medications




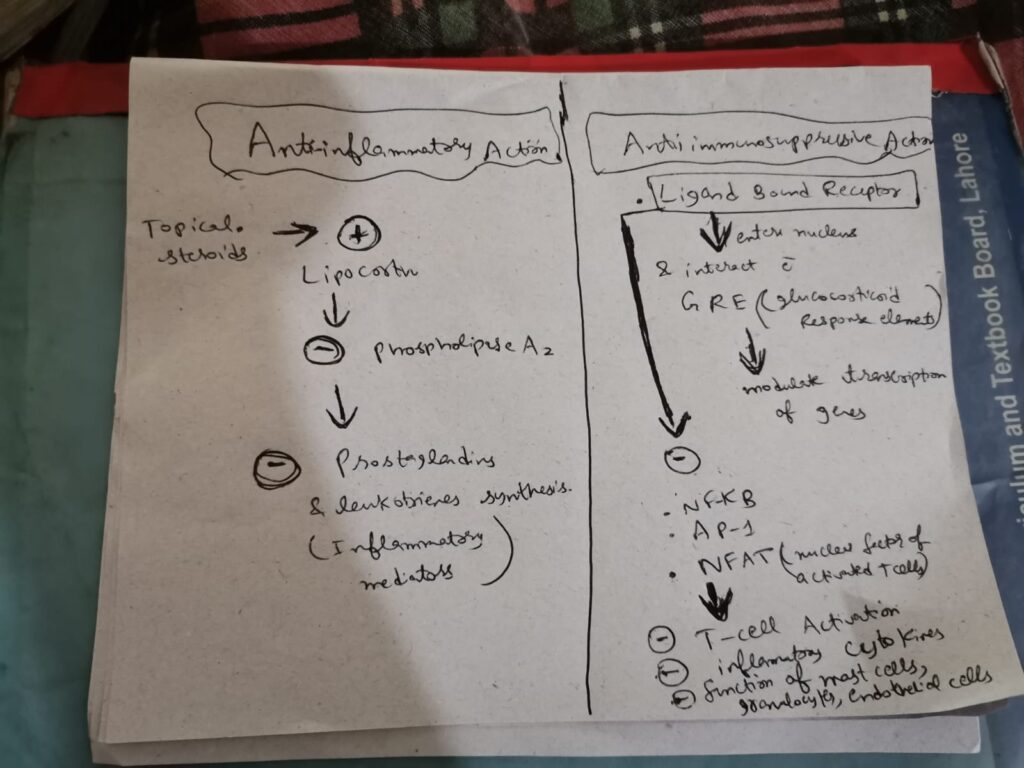
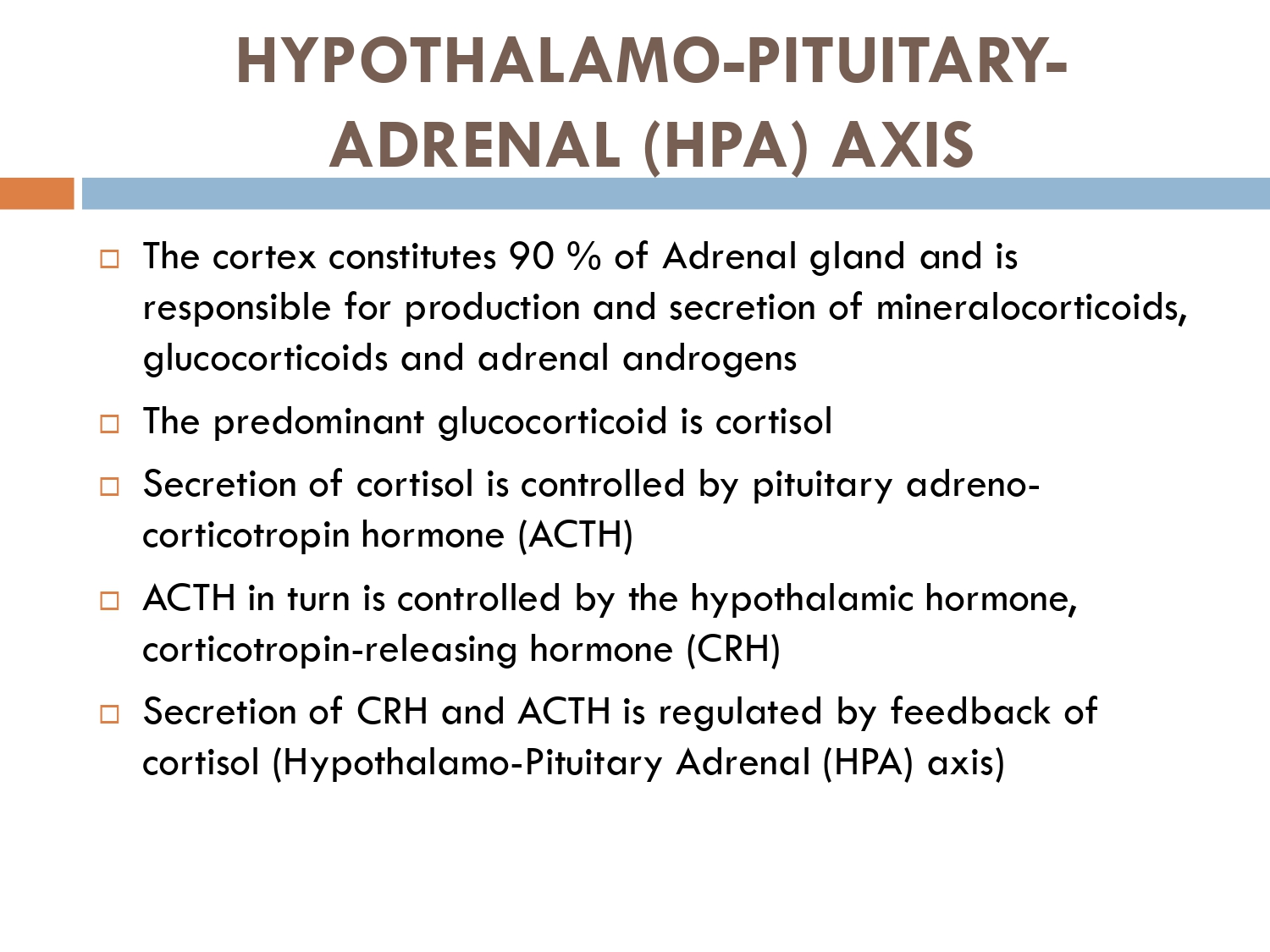
Glucocorticosteroids (GCS) are potent anti-inflammatory and anti proliferative agents widely used .



VASOCONSTRICTIVE EFFECT OF TOPICAL STEROIDS
corticosteroids are transcription factors, that induce synthesis of receptors for vasoconstriction.


Principles when initiating topical steroid therapy according to body area:
■ ■ Initiate lowest potency to control disease.
■ ■ Topical corticosteroids should be avoided on ulcerated or atrophic skin, and on skin with coexistent infectious dermatoses.
■ ■ Prolonged use of potent agent should be avoided.
■ ■ Treatment with low to medium potency preparations is recommended for large surface areas.
■ ■ Highly responsive diseases will usually respond to weak steroid preparations, whereas less-responsive diseases require medium potency or high-potency topical steroids.
■ Low-potency, ideally nonhalogenated, preparations should be used on the face and intertriginous areas.
■ ■ Very potent steroid therapy, frequently under occlusion, is usually required for hyperkeratotic or lichenied dermatoses and for involvement of palms and soles.
■ Because of the increased body-surface-area-to-body-mass-index ratio and increased risk of systemic absorption, high-potency preparations and halogenated–medium-potency preparations, should be avoided in infants and young children, other than for short-term application.
Dose titration of topical steroids
#Highly potent formulations should be used for short periods (2 to 3 weeks) or intermittently.
#Once disease control is partially achieved, the use of a less-potent compound should be initiated.
#Reduce frequency of application (eg, application only in the morning, alternate-day therapy, weekend use) once disease control is partially achieved.
#Sudden discontinuation should be avoided after prolonged use to prevent rebound phenomena.
#Special guidelines should be followed when treating certain body areas (eg, intertriginous areas) or certain populations (eg, children or the elderly) to prevent the occurrence of local or systemic adverse effects.
#Close monitoring and further evaluation is recommended if systemic absorption of corticosteroids is suspected.
#Use combination therapy when clinically indicated (eg, addition of topical calcineurin inhibitor, tretinoin or calcipotrien)
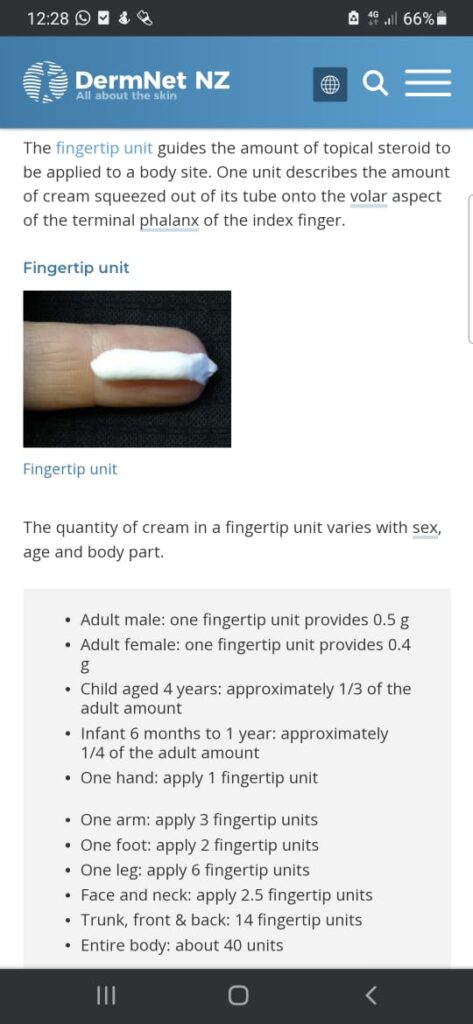
Remember rule of half for FTU application as follows
2% BSA =IFTU= 0.5 grams
And on average it covers 300cm squares area
💊 It is recommended that pts should use no more than 50 grams of super potent steroids/ week
💊 while for potent steroids no more than 100 grams/ week
👶 while in children and babies have a high ratio of surface area to body volume and are more vulnerable to pituitary-adrenal suppression as a result of systemic absorption.



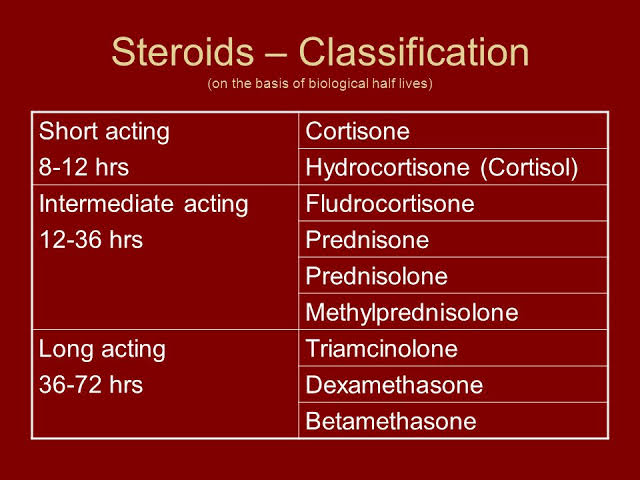
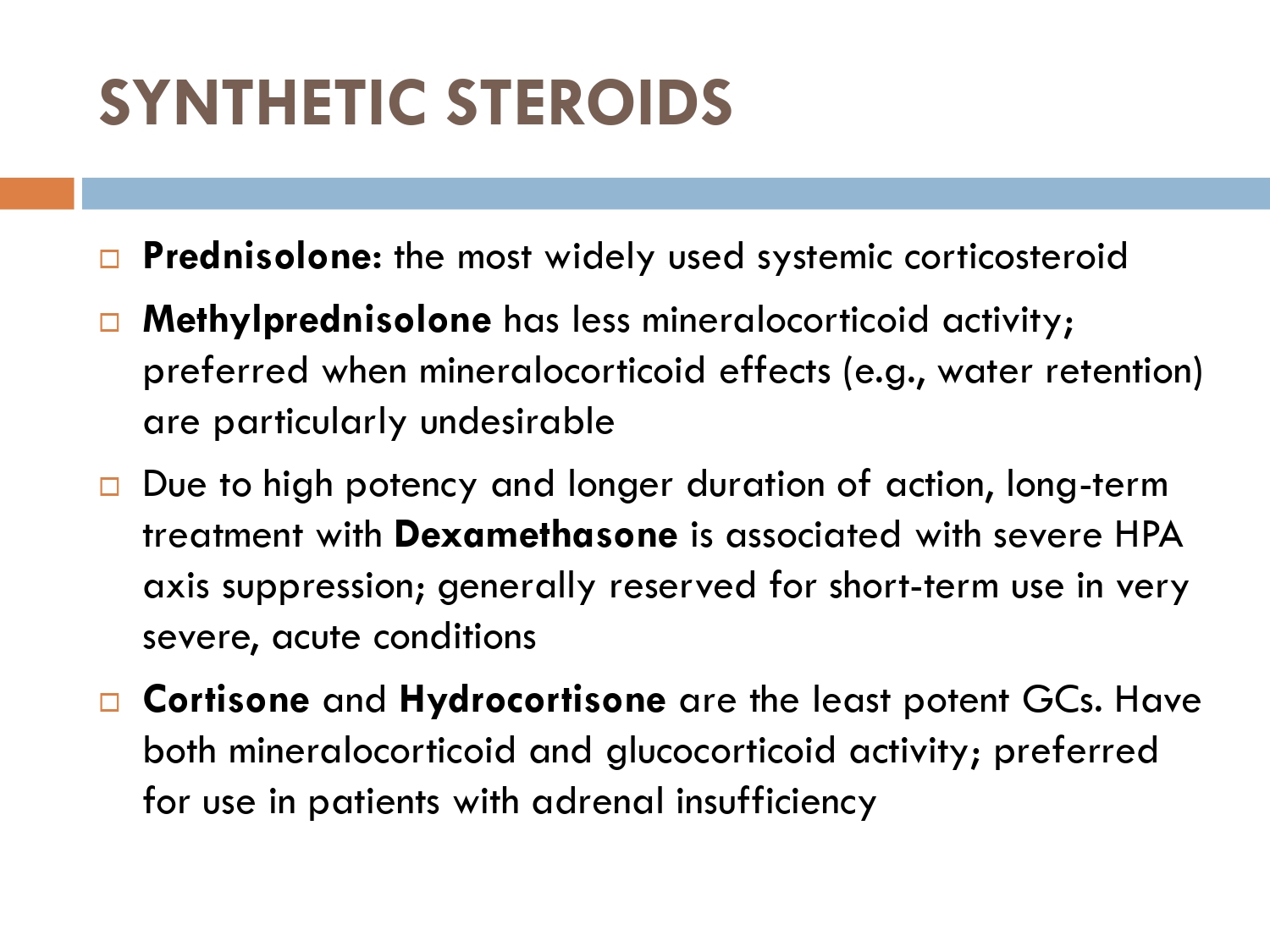
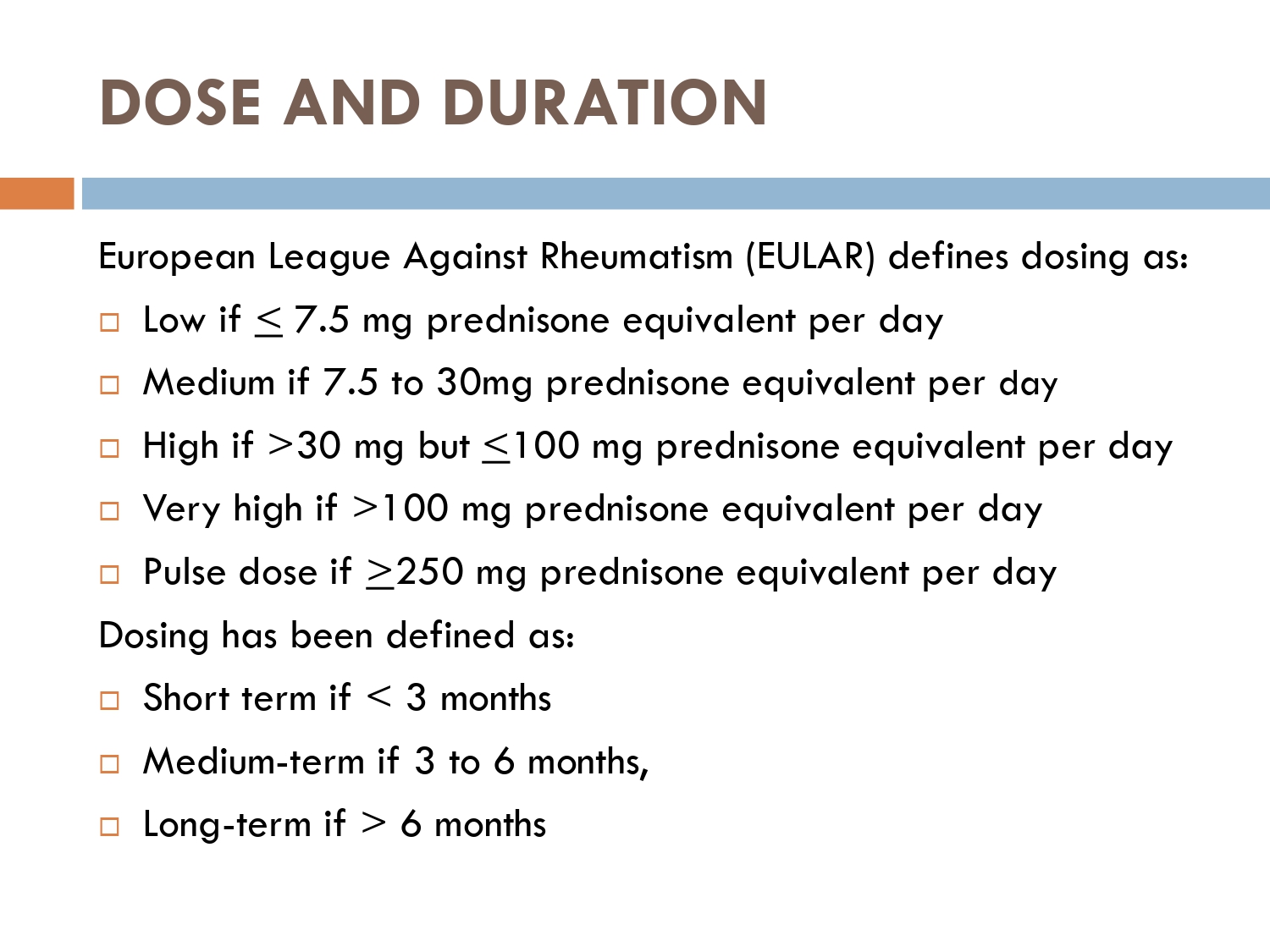
CLASSIFICATION OF SYSTEMIC STEROIDS :
1-GLUCOCORTICOIDS.
2-MINERALOCORTICOIDS.
3-ANDROGENS.
4-ESTROGENS.
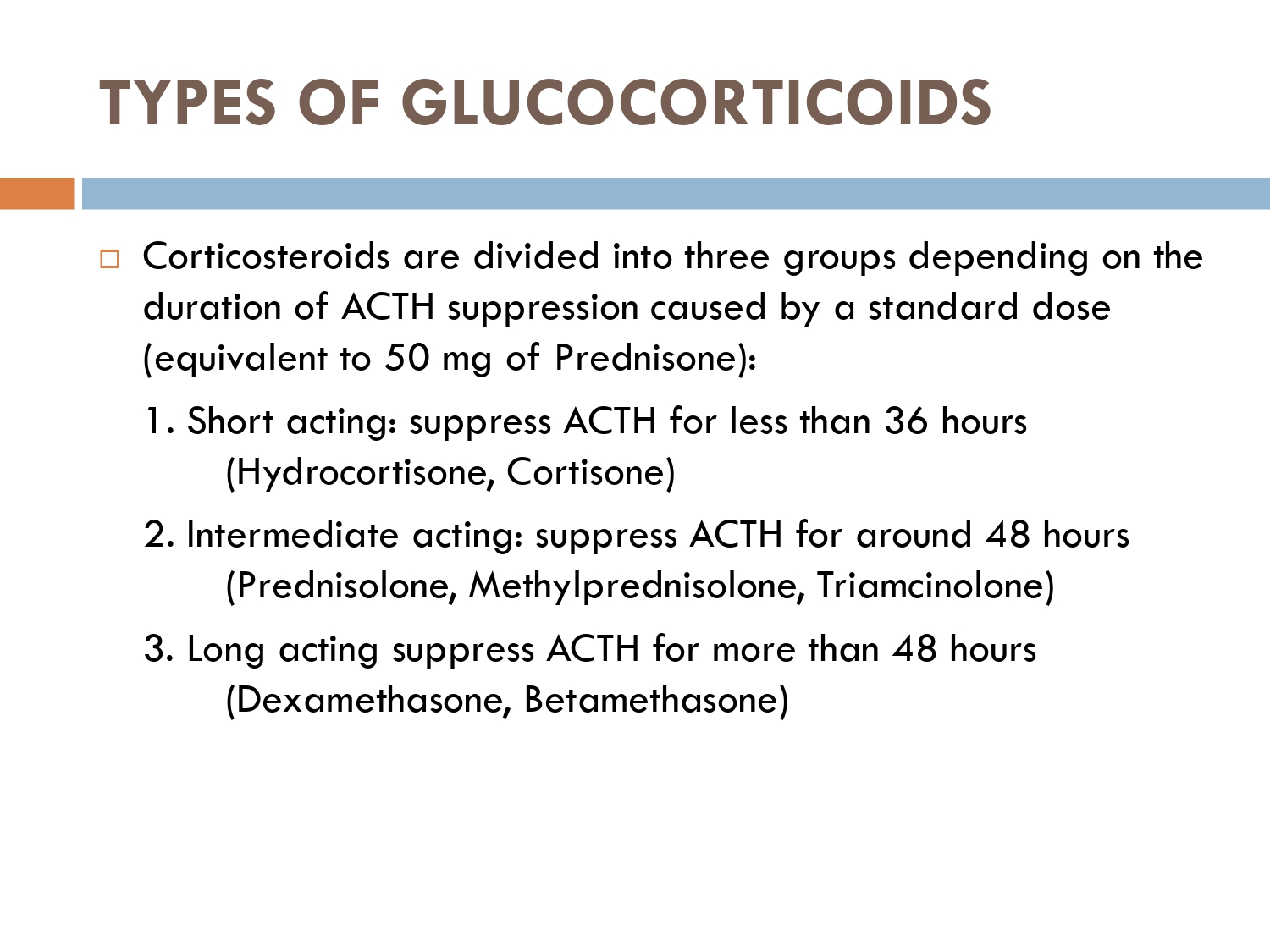
CLASSIFICATION OF GLUCOCORTICOIDS:
1-Short acting:
🚨Cortisol
🚨cortisone
2-intermediate acting:
🚨prednisone
🚨Prednisolone
🚨triamcinolone
🚨methylprednisolone
3-long acting glucocorticoids:
🚨Dexamethasone
🚨betamethasone
MINERALOCORTICOIDS
🚨Fludrocortisone
🚨Desoxycorticosterone acetate.
🔴 What are glucocorticoids?
🔅Glucocorticoids(GCs) are a family of steroid hormones that have vital immuno-modulatory , metabolic and anti-inflammatory actions.
🔅 Metabolic actions: breakdown of fats, carbohydrates and proteins.
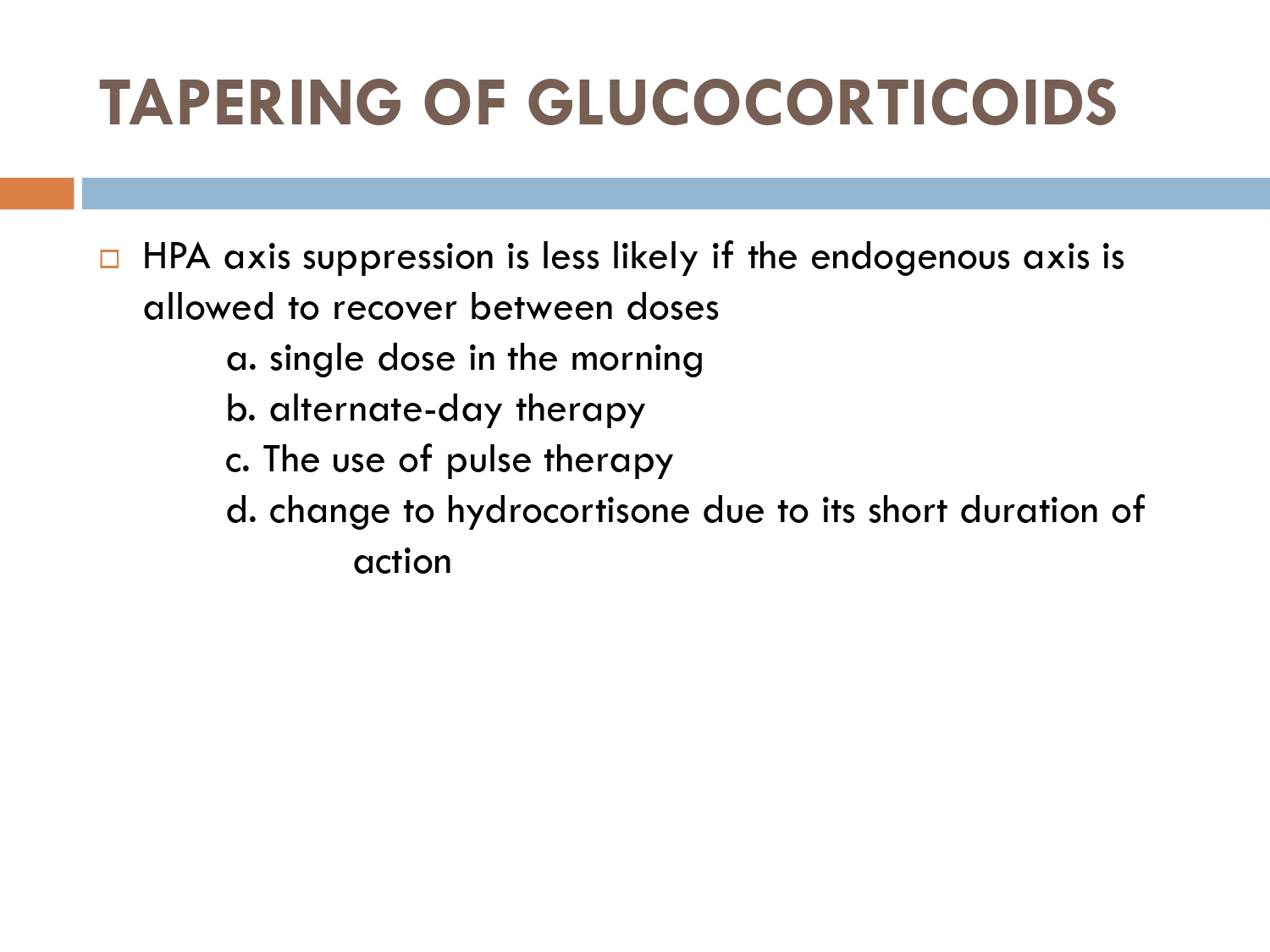
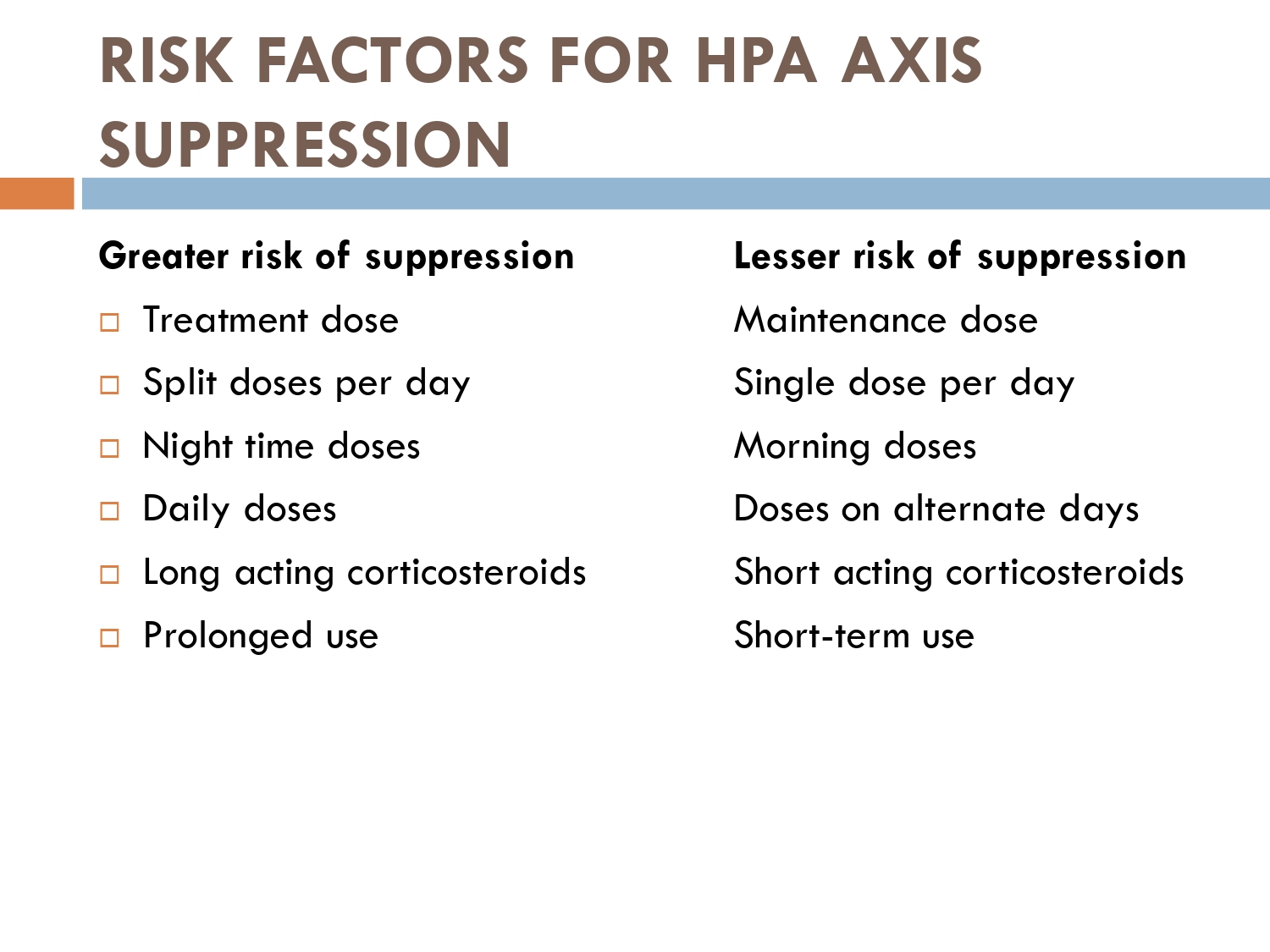
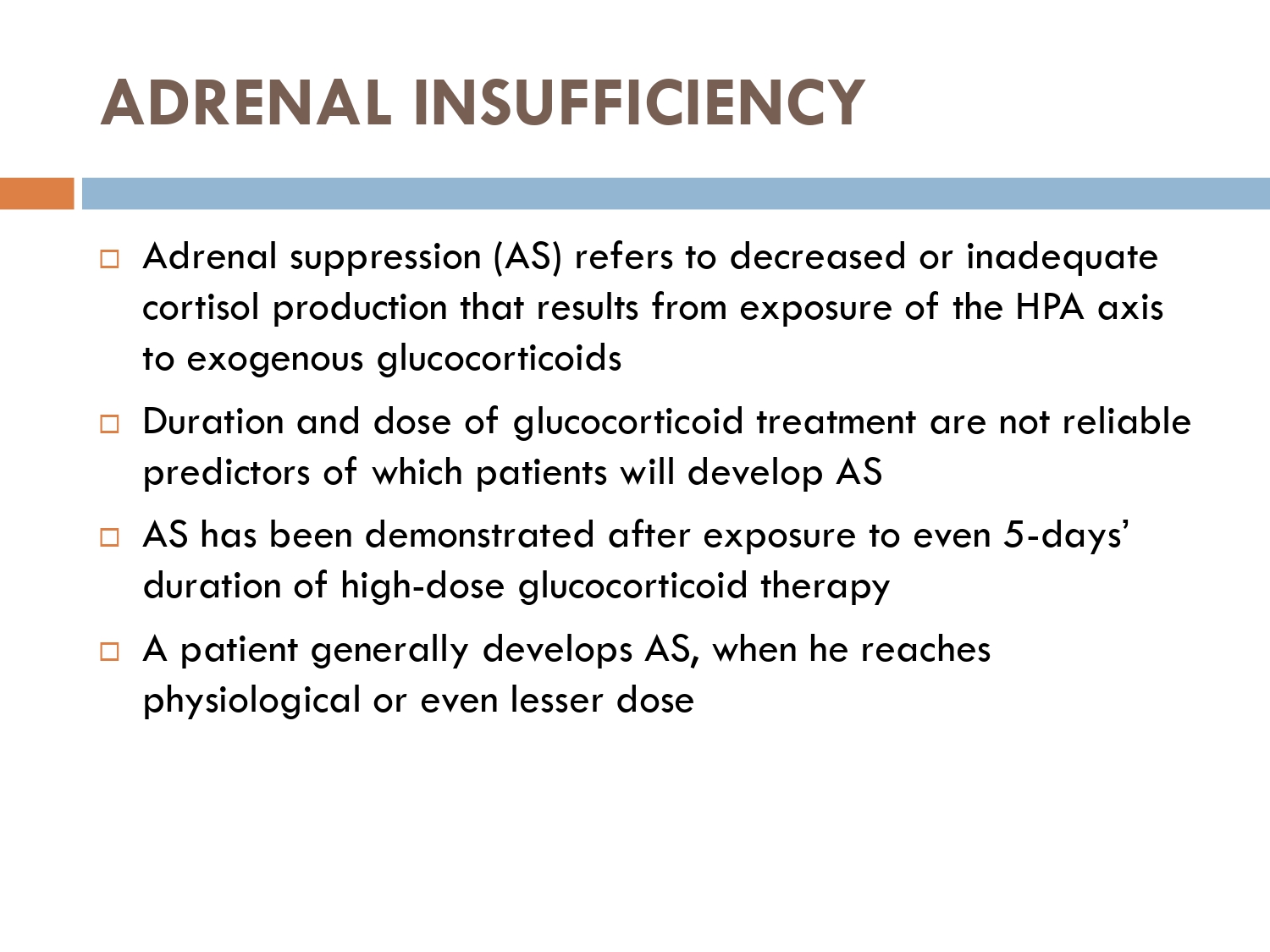
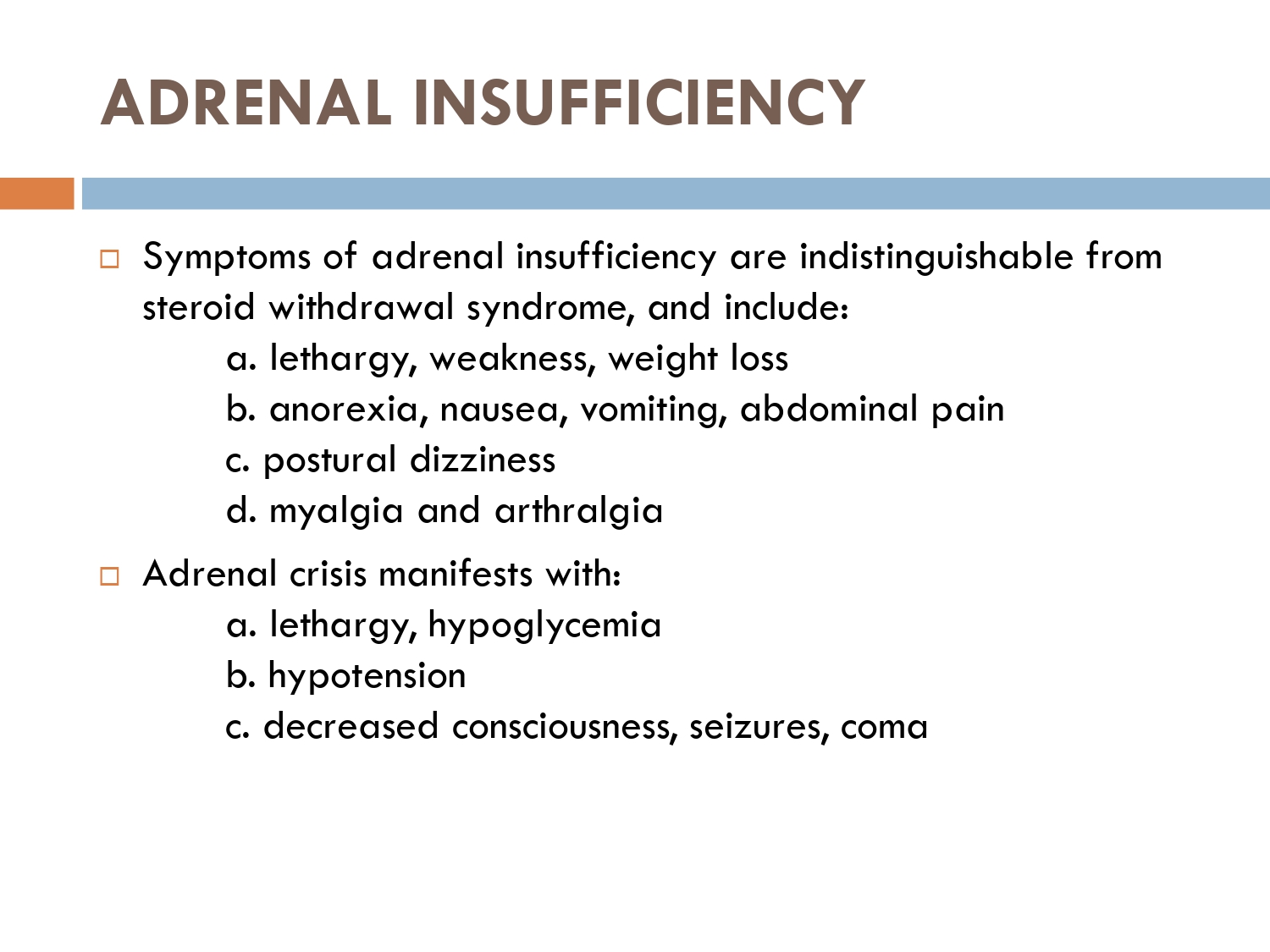
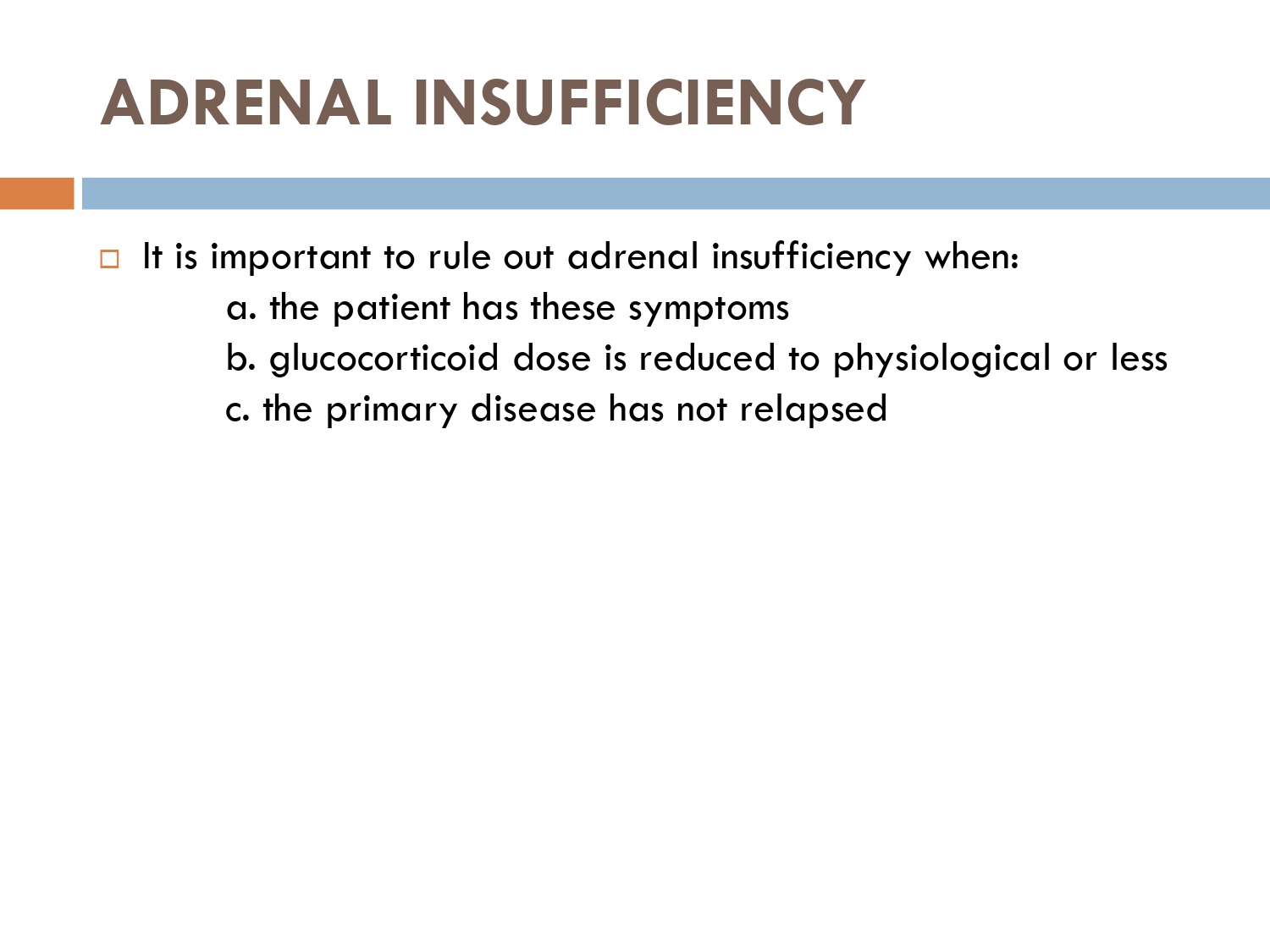
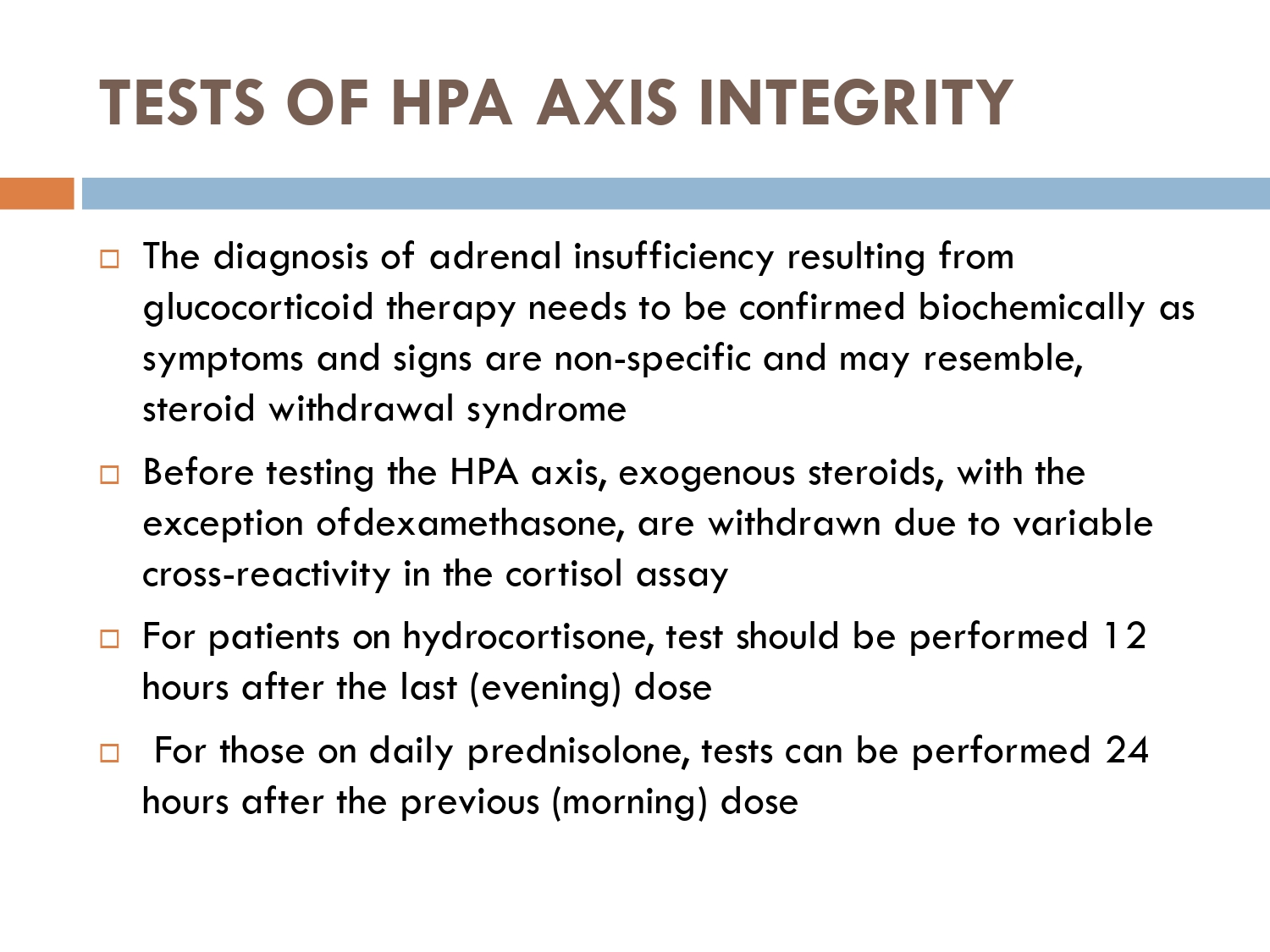
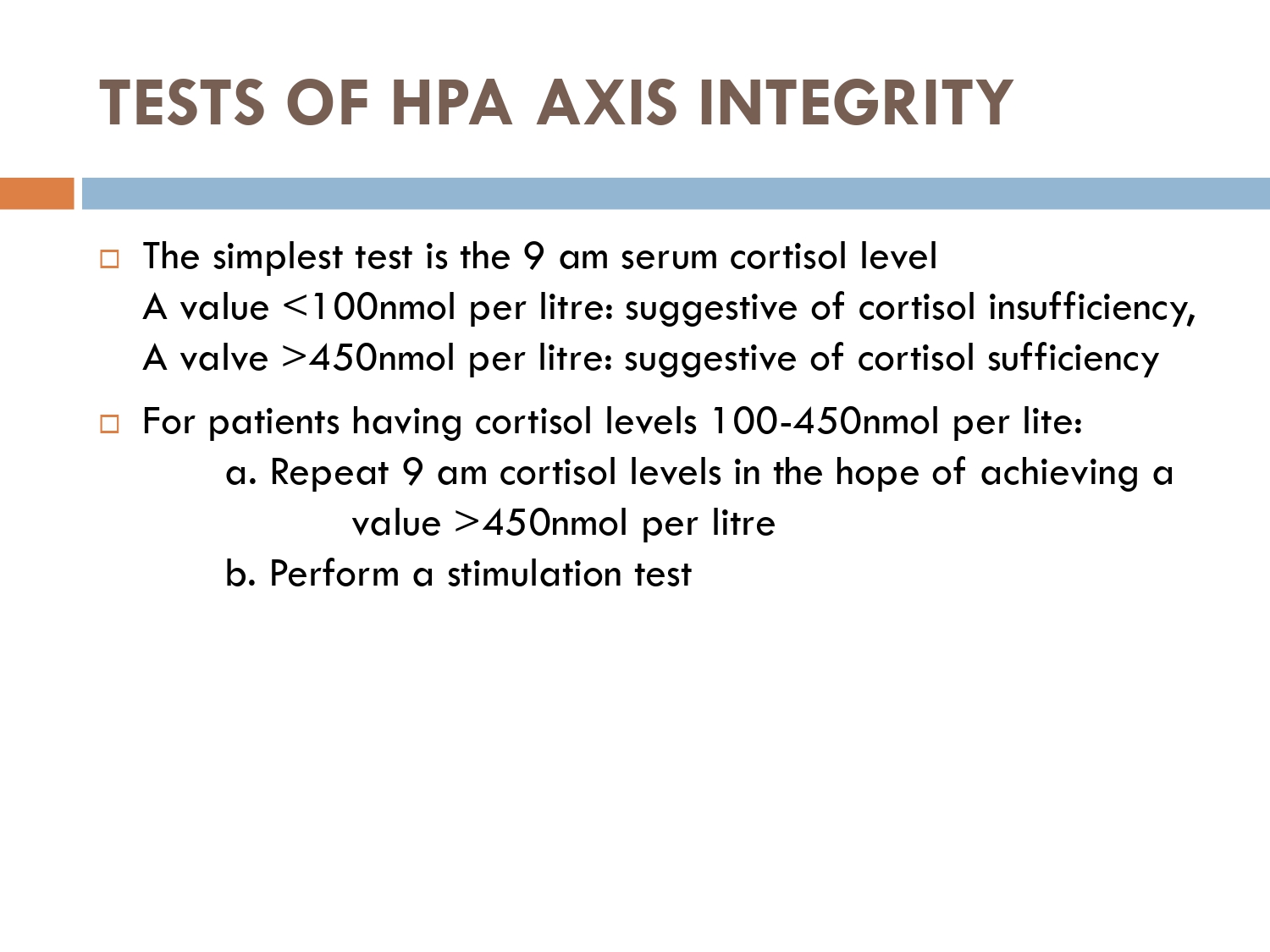
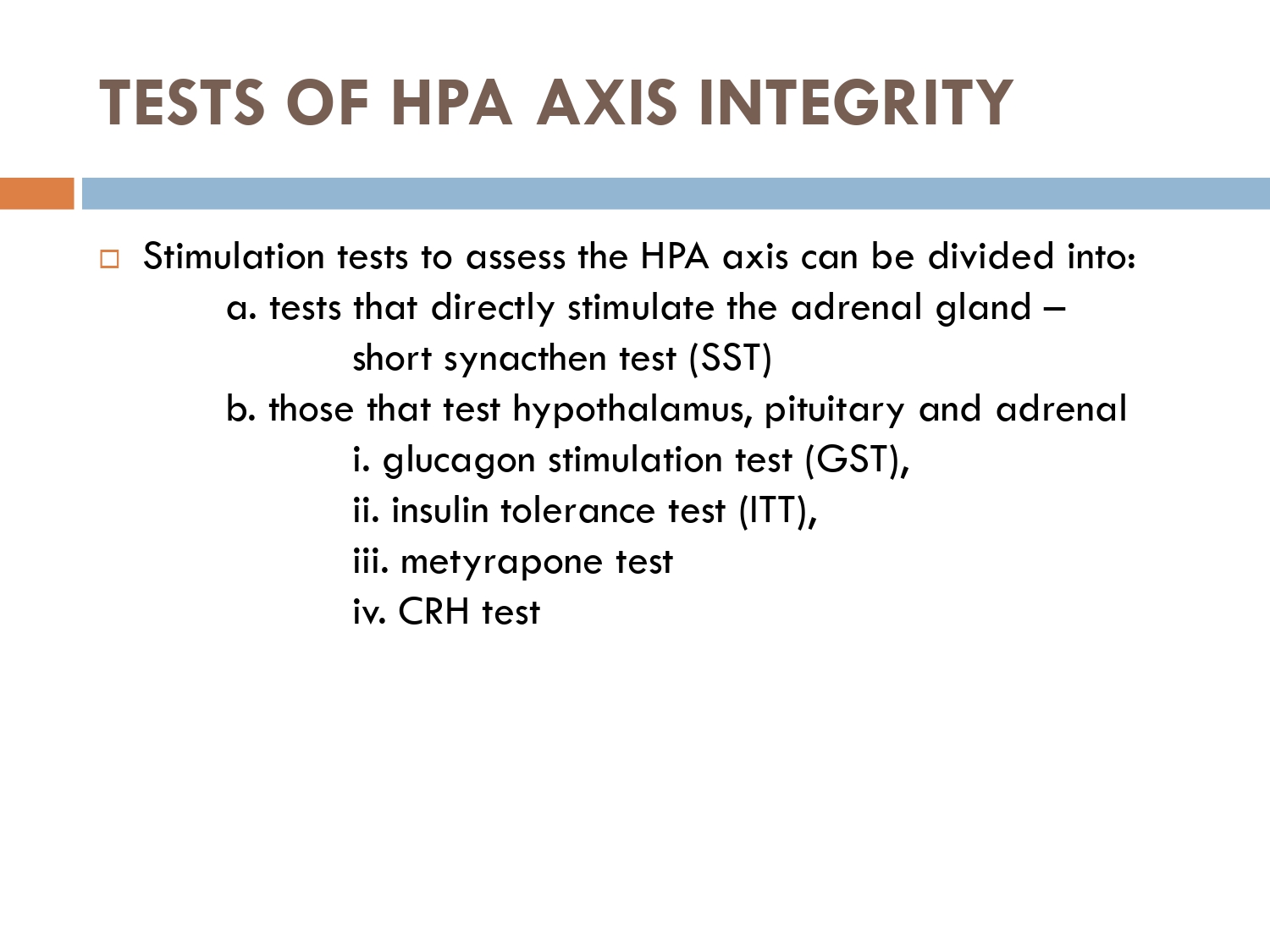
How to check HPA axis suppression
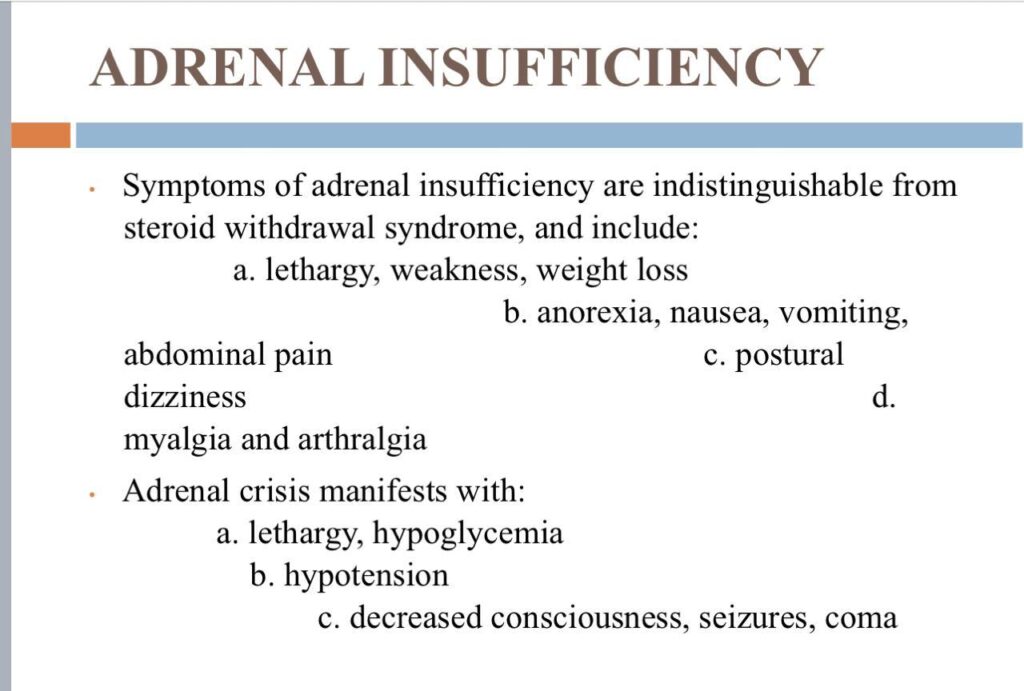
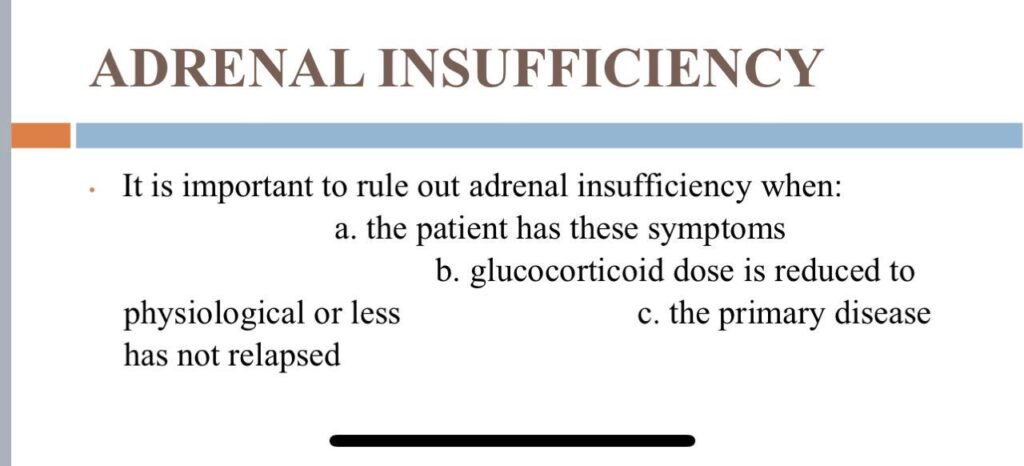
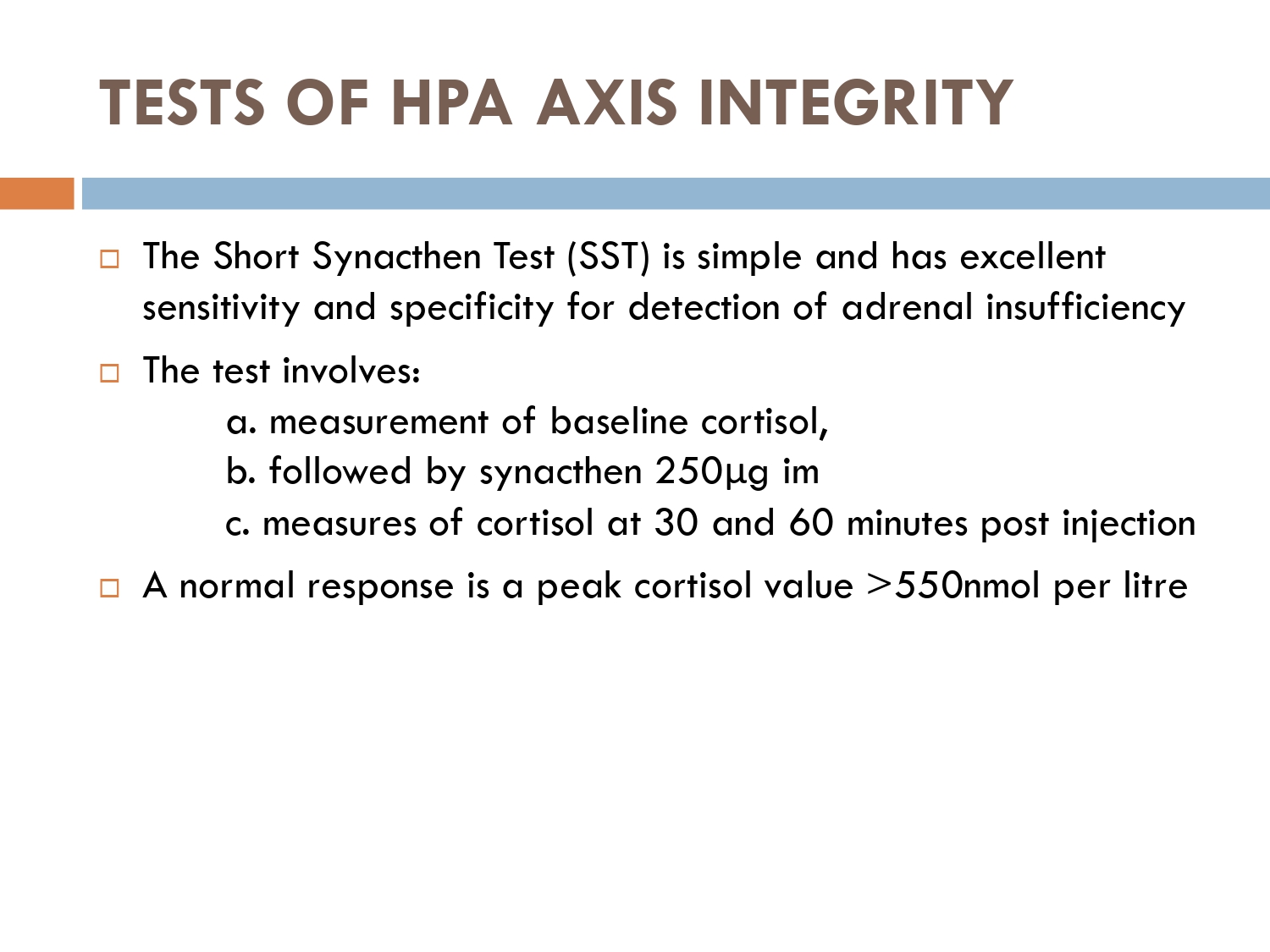
The early morning cortisol level and 24 h urinary cortisol level both provide a measure of basal HPA axis function for a patient on GCs, and the short synacthen stimulation test is a measure of the stress responsiveness of the adrenal: these are an option for assess- ing adrenal reserve towards the end of long‐term GC therapy.
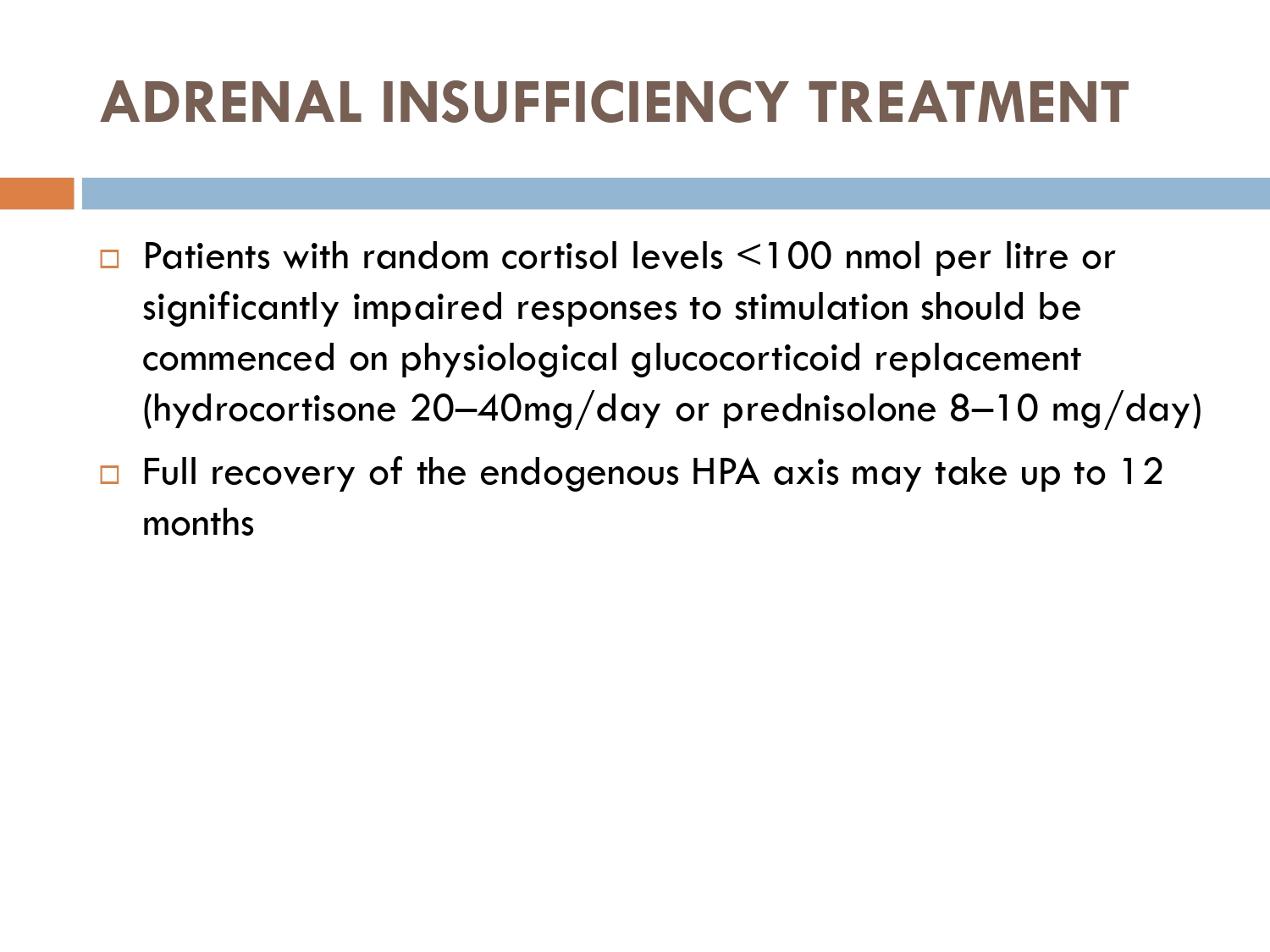
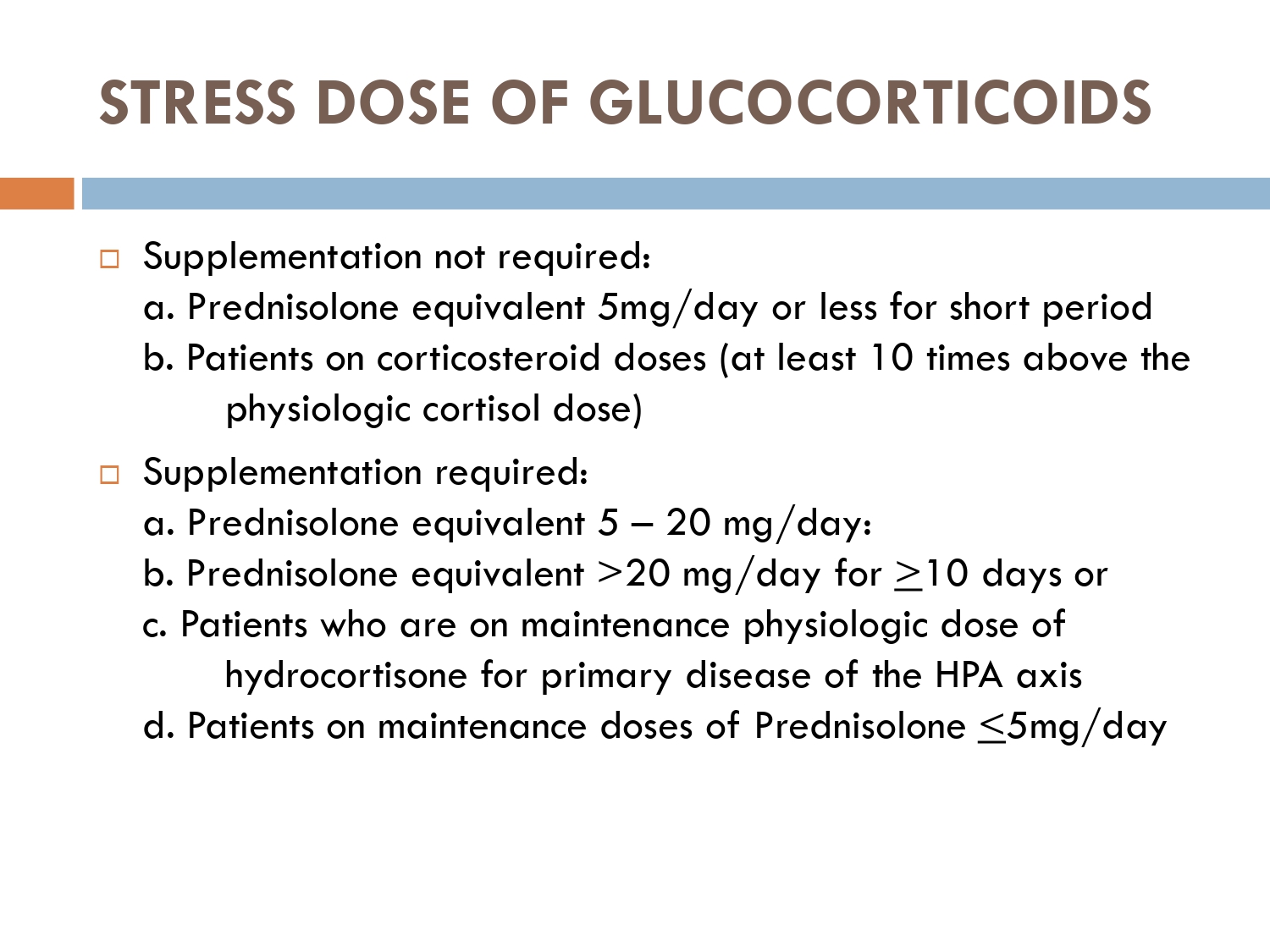
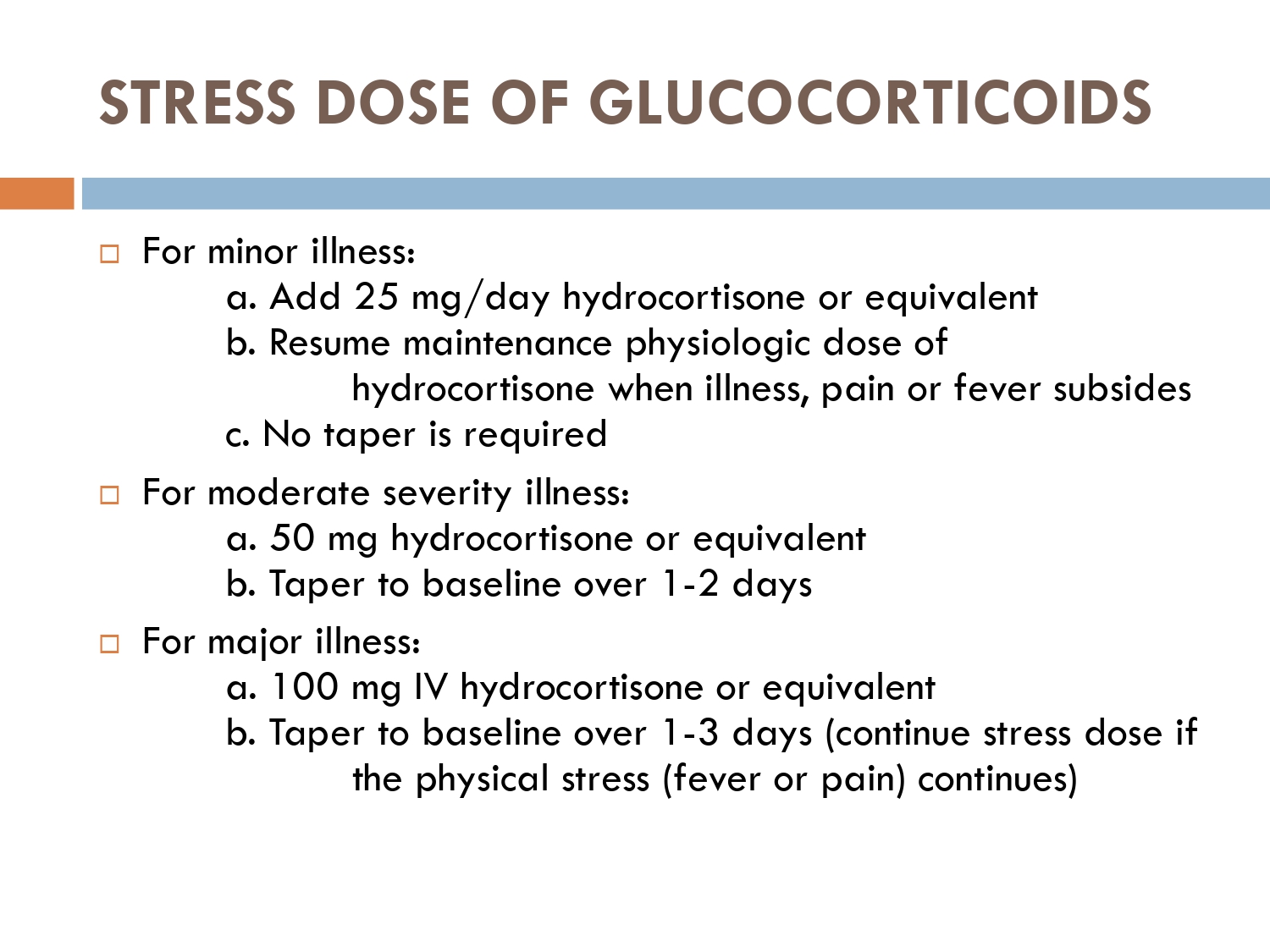
How to prevent
>GCs taken in a daily dose of more than 40 mg for more than 1 week (or a lesser dose for more than 3 weeks) should be tapered gradually [2]. >Intercurrent illness, infection, surgical procedure or trauma requires a temporary increase in GC dose or, if a prolonged GC course has recently been completed, a temporary reintroduction of GC to compensate for a diminished adrenal response. >Anaesthetists must know if their patient is taking or has been taking a GC within 3 months of surgery [2]. >Patients on long‐term GC therapy should carry a steroid treatment card with them at all times.
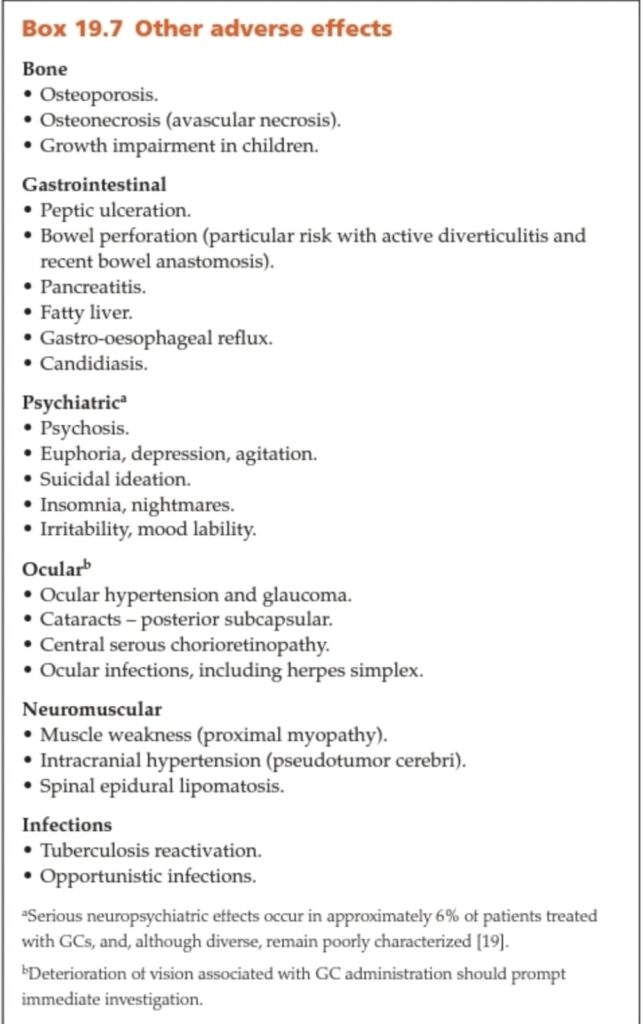
Adverse effects
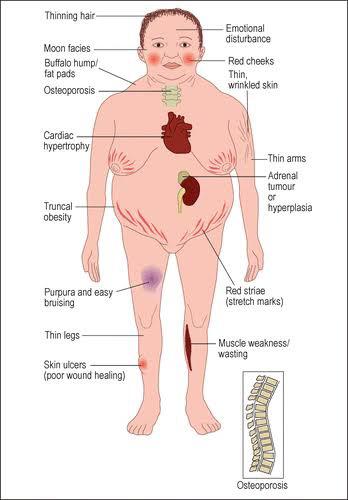
Cushingoid features:
Cutaneous:
– thining of hair
– Acne
– red cheeks
– moon facies
– buffalo hump/fat pad
– acanthosis nigricans
– thin wrinkled skin
– thin arms and legs
– truncal obesity
– red striae
– purpura and easy bruising
– skin ulcers
MSK:
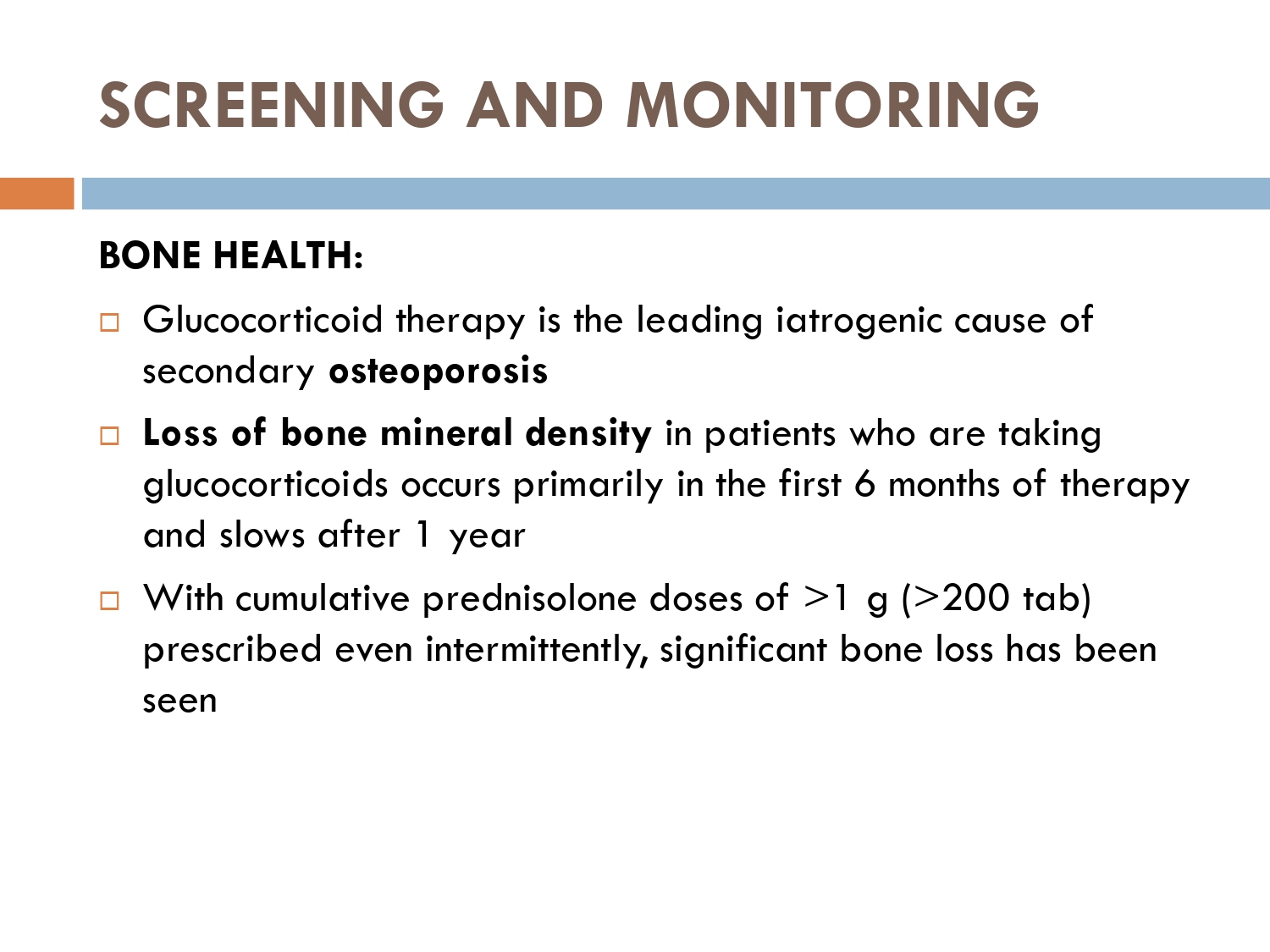
– osteoporosis
– muscle weakness and wasting
Psychological
– depression
– anxiety
– irritability
– loss of emotional control
– cognitive difficulties
General:
– HTN
– DM
– increased infection
Bacterial infections: cellulitis, wound infection
Fungal infections: tinea, candida, pityriasisversicolor
Viral infections: herpes zoster
– fatigue
– amenorrhea
– impotence
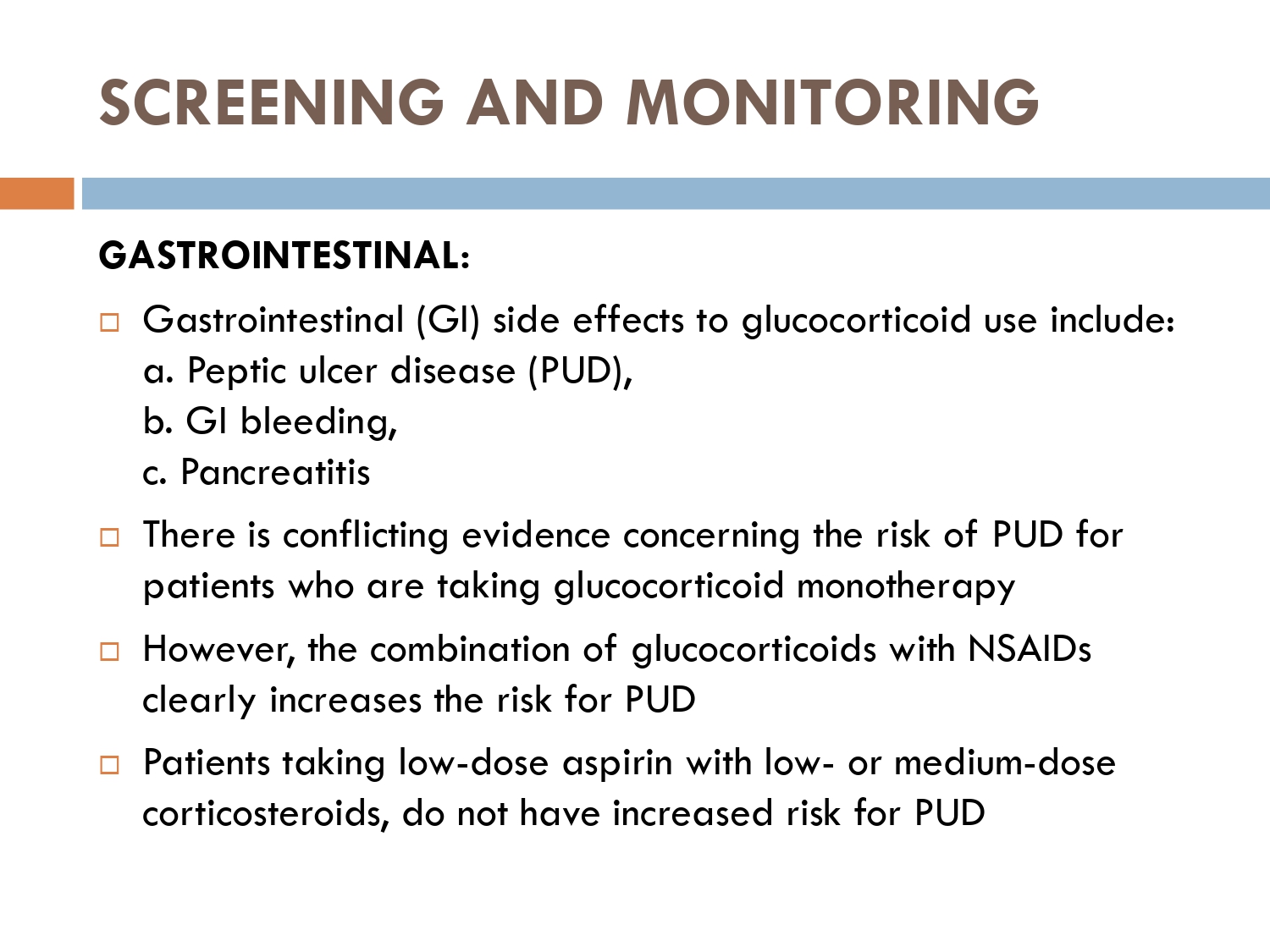
Contraindications of systemic steroids
Relative contraindications
■ Systemic infections ( fungal, viral, bacterial)
■ Tuberculosis
■ Uncontrolled Diabetes
■ Uncontrolled Hypertension
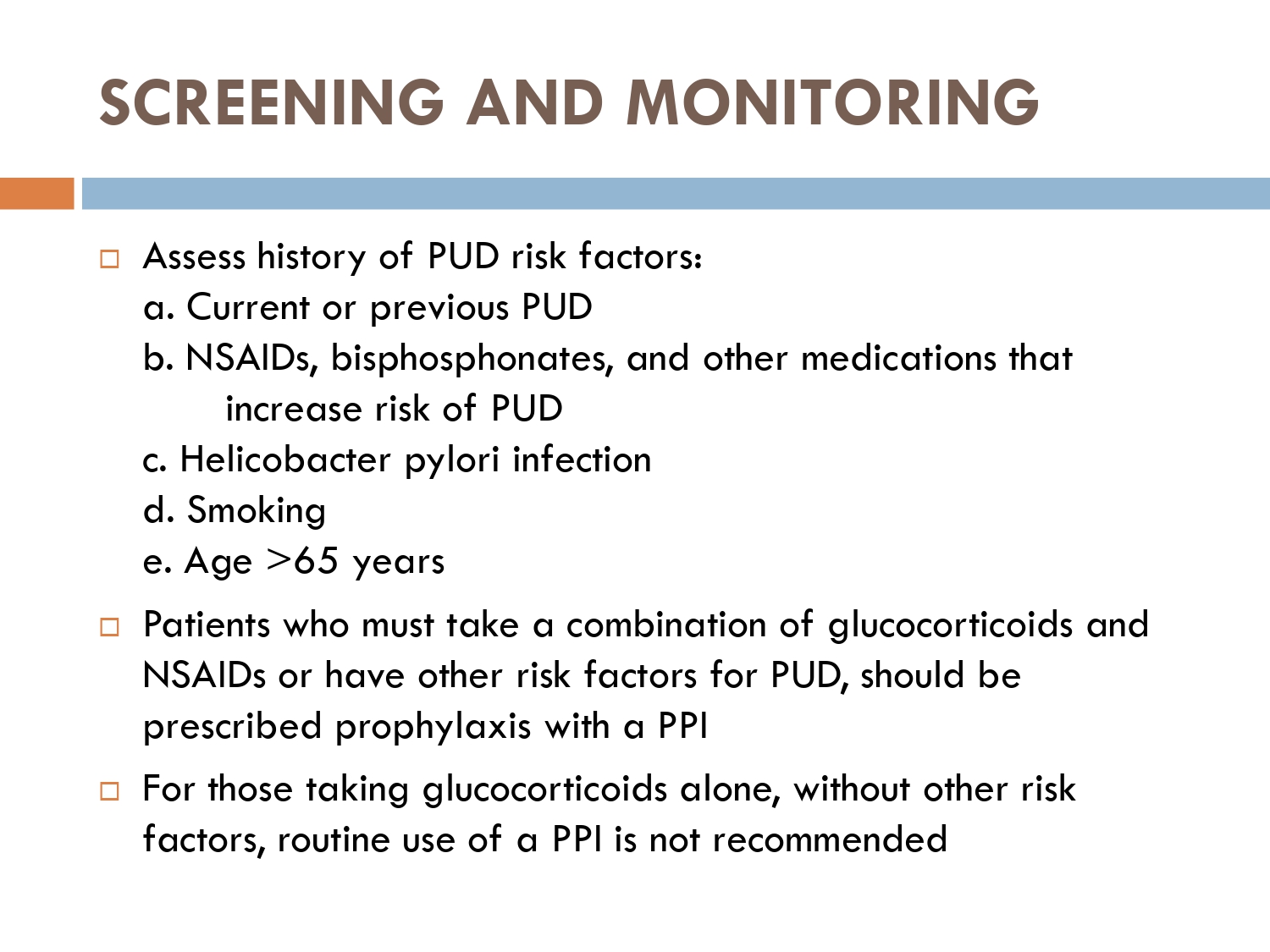
■ Peptic ulcer disease
■ Osteoporosis
■ Psychosis
■ Renal failure
■ Heart failure
■ Live attenuated vaccines
Drug drug interactions:
– GC antagonize hypotensive effects of ACE inhibitors, alpha blockers, Angiotensin-II receptor antagonists, beta blockers, CCB and clonidine
– Antagonize the oral hypoglycemics
– Antagonize diuretics and increase risk of hypokalemia
– increase the risk of gi bleeding when given with NSAIDS
– Antacids decrease steroid absorption
– Anticonvulsant reduce plasma levels of GCs
– Estrogen containing ocp increase plasma conc of GCs
– Variable effect on anticoagulant action of coumarins
– Increase intracranial pressure when used with tetracycline and retinoids
– GCs may impair immune response to vaccines
Dermatological uses of glucocoticoids
- Autoimmune Bullous disorders
- Connective tissues disorders
- Vasculitides
- Neutrophilic dermatosis
- Eczematous disorders
- Papulosquamous disorders
7.urticaria
- Sarcoidosis
- Type 1 lepra reaction
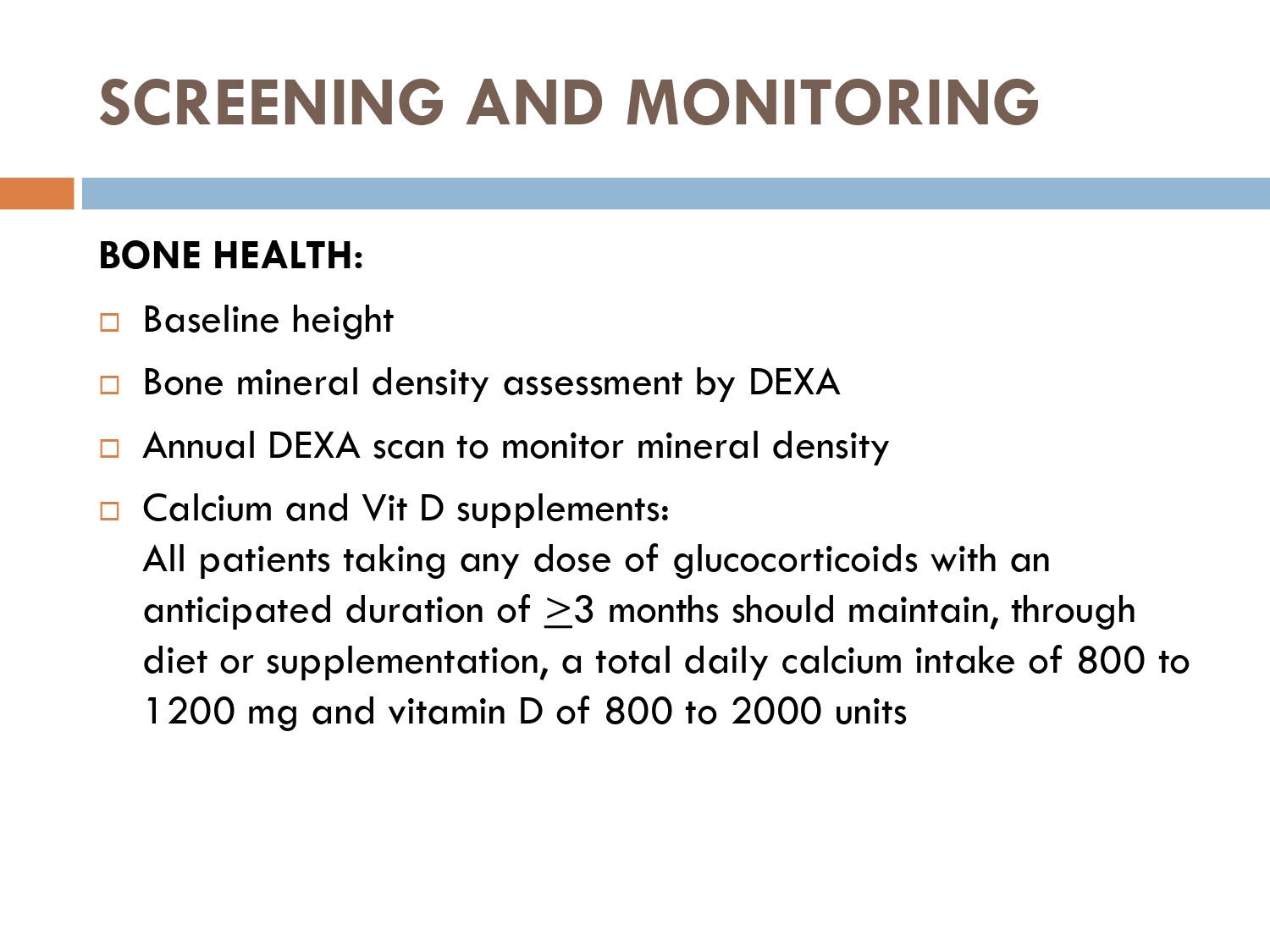
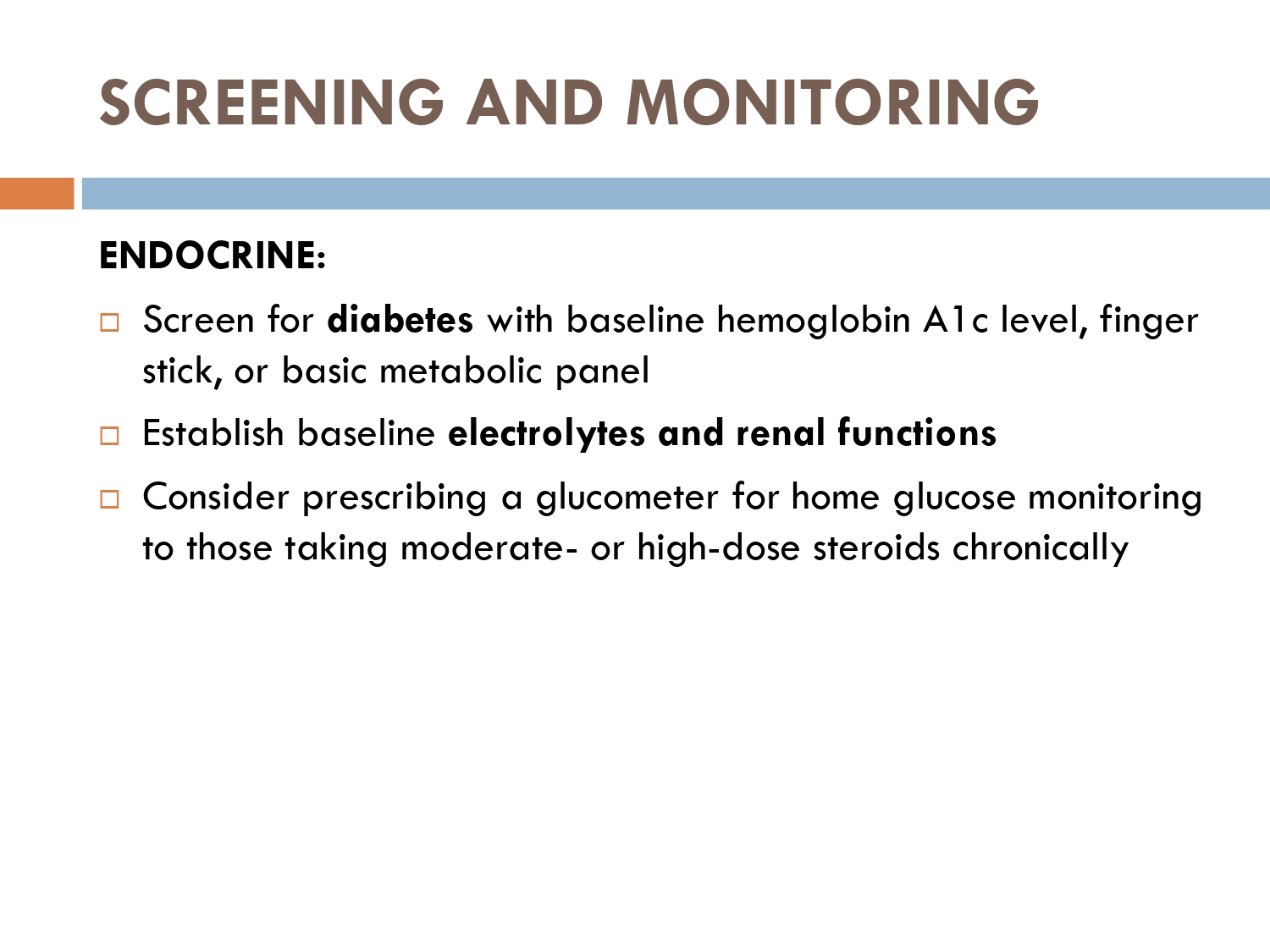
Baseline measures of:
body weight and height blood pressure
CBC
serum electrolytes and rfts
RBS/FBS/HbA1c
Lipid profile
hep B/C
HIV serology
MT (if +ve then CXr and IGRA)
Bone mineral density via DEXA scan
stool for strongyloides stercolaris
ophthalmological examination for cataract and intraocular htn
Prior to initiating GC therapy, the patient and family members should receive appropriate education, in particular about the potential adverse effects and the monitoring details.
A steroid treatment card should be provided and the information on it kept up to date.
monitoring :
follow up patients at 1month and then every 2-3months
at each visit enquire about
weight
height(in children)
B.pressure
electrolytes
FLP
FBS
eye examination at every 6-12months
dexa scan annually
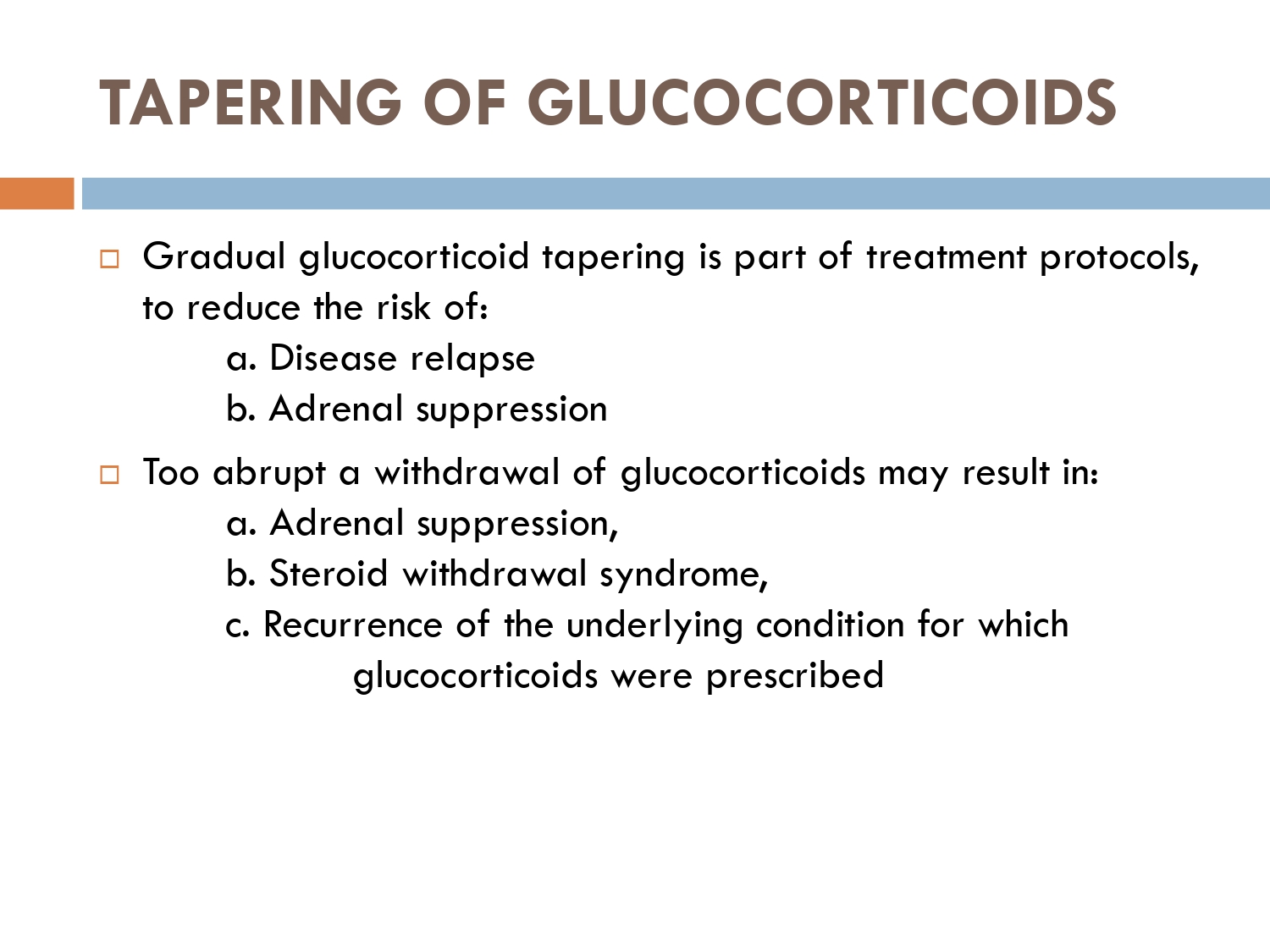
tapering of steriod
American academy of dermatology journal
for patients taking steriods>40mg/prednisolone
taper 10mg/week until 40mg daily..remain on 40mg for a week
then(at prednisolone 40mg/day)
taper 5mg/week till 20mg /day of dose
stay on 20mg predni daily for a week
then(at prednisolone 20mg/day)
2.5mg/week to dose of 5mg/day
stay on 5mg for a week
then (at prednisolone 5mg/day)
taper 1mg/week
continue till pt is off steriods
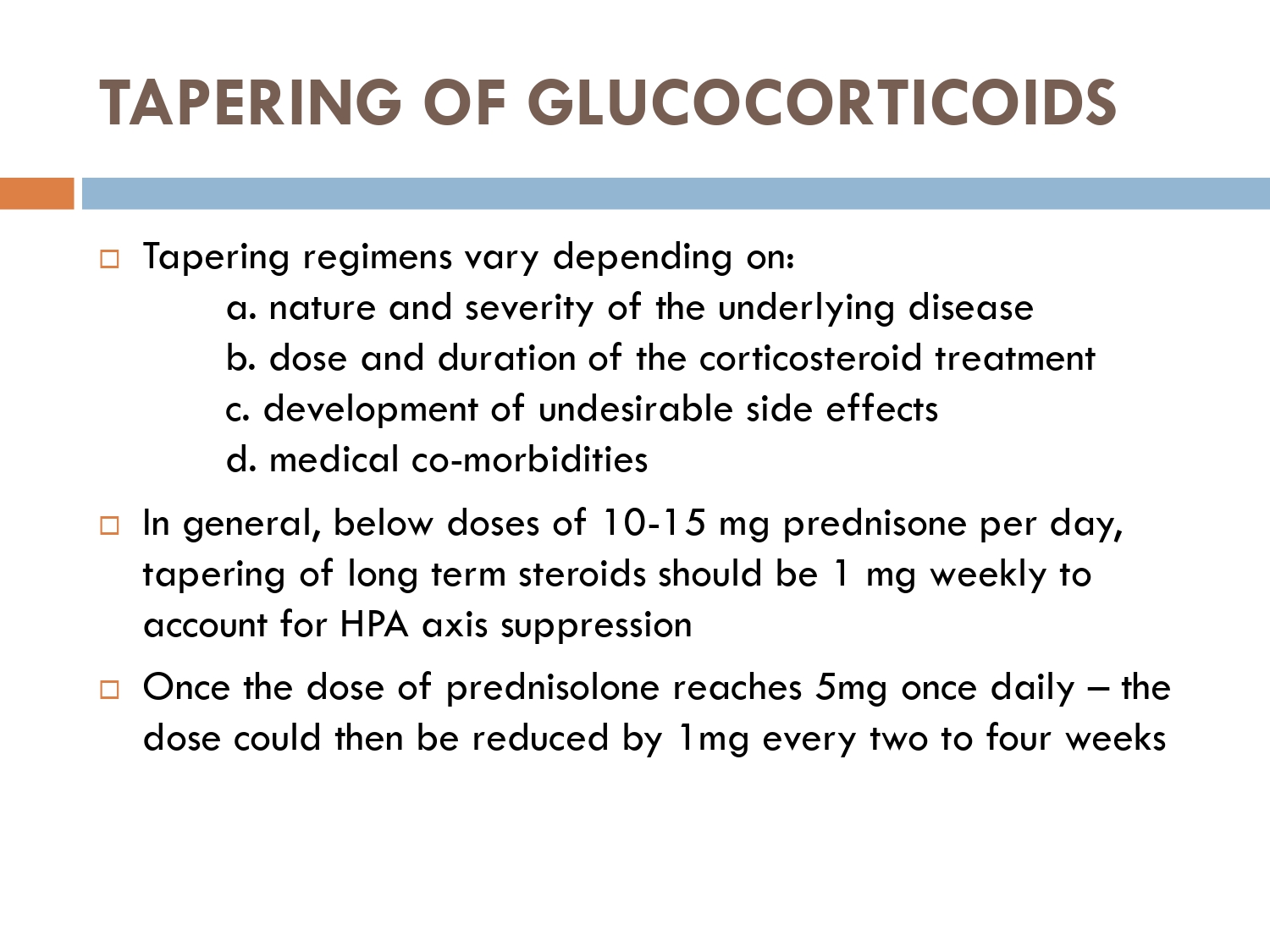
According to American guidelines if pt is on more than 40 mg/ day then taper 10 mg/ week until pt is on 40 mg/ day,and keep 40 mg/ day for a week
If starting dose is 40 mg/ day then taper 5 mg/ week until pt is on 20 mg/ day
And stay on 20 mg/ day for a week
If starting dose is 20 mg/ day then taper 2.5 mg/ week to a dose of 5 mg/ day and keep on 5mg/ day for a week
If pt on 5mg steroids/ day
Taper 1mg/ week couuntinue tapering till pt is off the steroids
According to BAD guidelines If pt is more than 60 mg steroids/ day taper 10mg/ every 3 weeks till pt is on 60 mg/ day
If pt is on more than 30 mg/ day then taper 10 mg every month till pt is on 30mg/ day
If pt is on below 30 mg steroids/ day then taper 5mg every 3 months till pt is 20mg day
If pt is on below 20 mg/day
Taper 2.5 mg every 3 months till pt is off the steroids
According to BAD 2017 guidelines
Taper steroids by 5-10 mg or 50% reductions in biweekly manner
When pt is on less than 20 mg/ day then taper more slowly with 2.5 mg / week for 2 to 4 weeks till pt is on 10mg/ day
If pt on 10 mg then reduce 1mg/ kg on alternate days till pt is off steroids
Brands:
Tab deltacortil-prednisolone
Tab Betnesol-betamethasone
Inj solucortef-hydrocortisone
Inj decadron-dexamethasone
Inj Triton, k-cort- triamcinolone
In oral steroids enteric coated tablet is also available with name of Rapicort.with least git side effects.












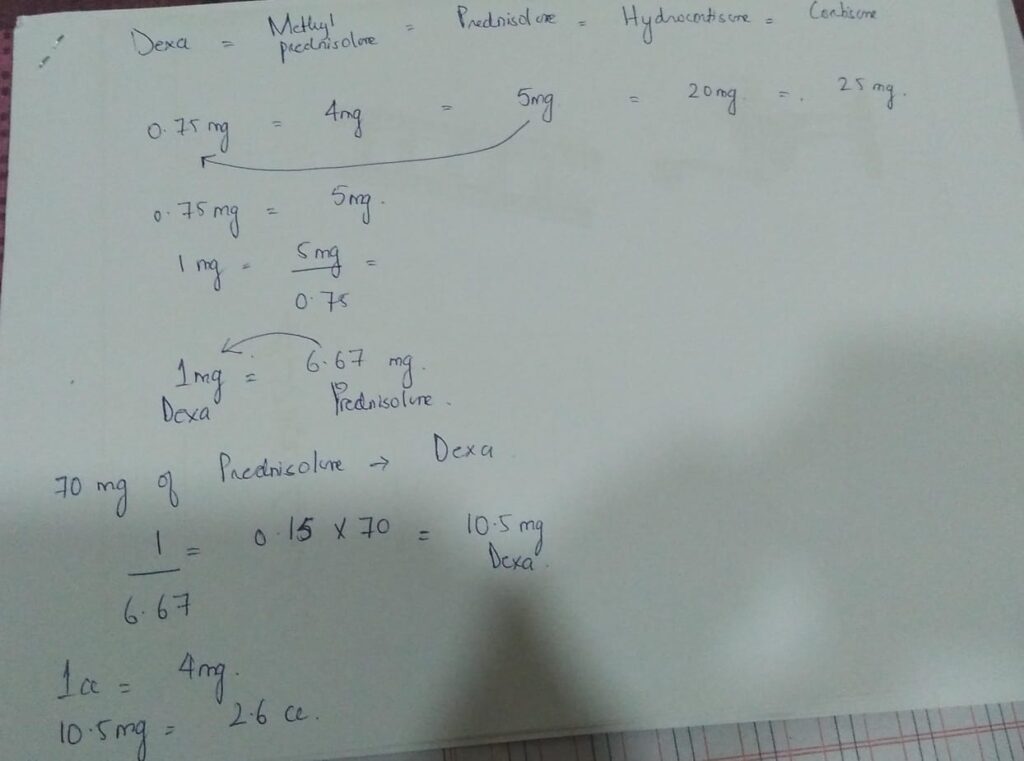
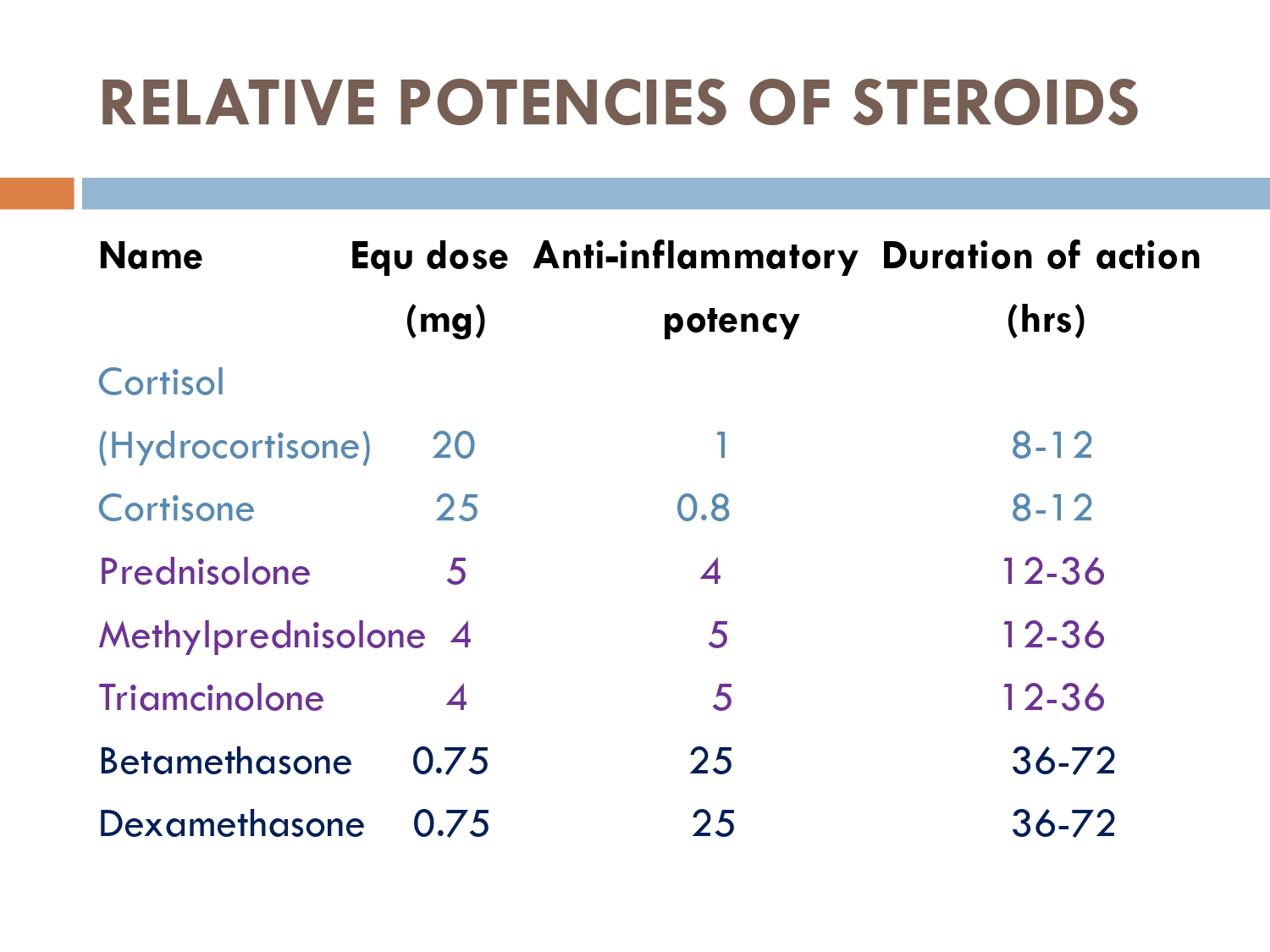
0.75(dexa) most potent
4mg( methyl prednisolone
5mg(prednisolone/
deltacortil)
20mg( Hydrocortisone)
25mg ( cortisone)
70 mg prednisolone=10.5mg dexa
1cc =4mg dexa
2.6cc= 10.5mg Dexa
The American College of Rheumatology recommend
Calcium 1000 to 1,500 mg
1000 to 2000 IU of vitamin D supplements each day
in patients taking oral steroids
Annual dexa scan should also be done
What if the patient has renal failure? Will we give the same drug?
Cac plus qalsan d have cholecalciferol which is inactivated form vitamine d 3 it wont work in a patient with kidney failure as it requires activation in the kidney so we prescribe activated forms such as bonone (alfacacidiol) in such patients
It will be given prophylactically in patients with ckd particularly in patients at risk for vitamin D deficiency.
With long term use it will cause hyperphosphatemia for which nephrology team checks on pth levels regularly.
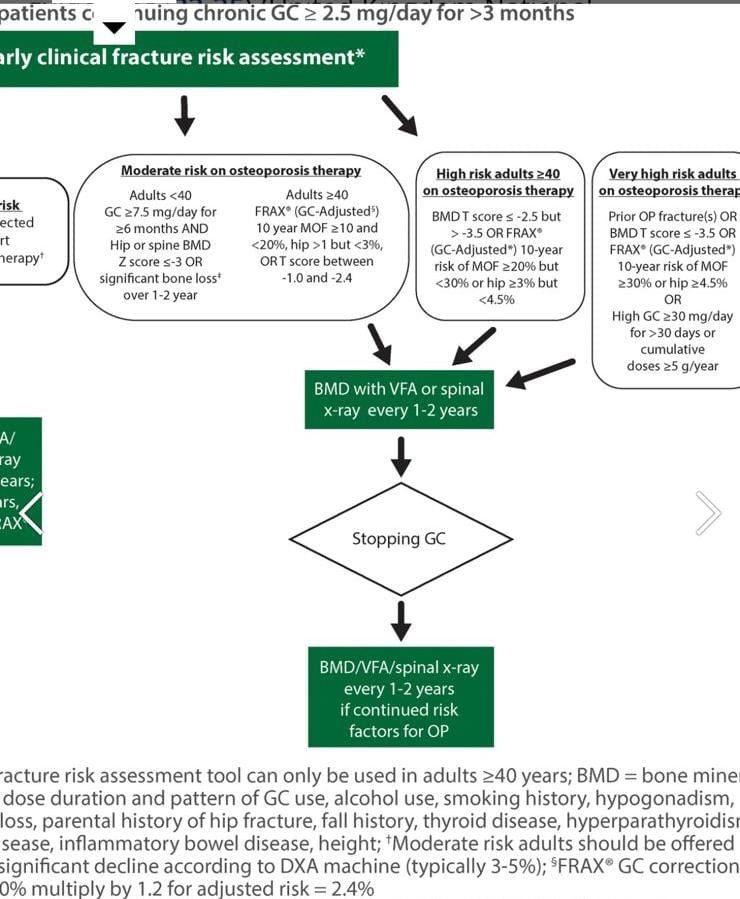
Pharmacologic Therapy Guidelines for Postmenopausal Women and Men Aged ≥40 Years:
- *Who Should Receive Therapy:*
– *High Risk of Fracture:* Postmenopausal women and men aged ≥40 years with moderate to high fracture risk should receive pharmacologic therapy.
– *Osteoporosis:* Men aged ≥40 and postmenopausal women with osteoporosis (previous fragility fracture or a BMD T-score ≤-2.5) should receive therapy.
– *Moderate Risk:* Those with T-scores between -1.0 and -2.5 who are at moderate or high fracture risk and are on glucocorticoids should consider therapy.
- *Glucocorticoid Use:*
– *Long-Term Use:* For those with T-scores between -1.0 and -2.5 and a lower fracture risk but on ≥7.5 mg/day of prednisone for ≥3 months, therapy is suggested.
– *High Dose:* Those on ≥30 mg/day of prednisone for >1 month should consider therapy even without other risk factors.
Fracture risk is calculated by frax calculator
Pharmacologic Therapy Guidelines for premonapusal women and men.
- *General Approach:*
– *Individualized Therapy:* Treatment should be tailored for eugonadal men and women based on their moderate to high fracture risk.
- *Premenopausal Women:*
– *With Fragility Fracture:* Women with normal ovarian function and a fragility fracture while on glucocorticoids should receive pharmacologic therapy.
– *With Accelerated Bone Loss:* Women without a fragility fracture but experiencing accelerated bone loss (≥4% per year) or a Z-score <-3.0 while on 7.5 mg prednisone equivalent for ≥3 months should also receive therapy.
– *High-Dose Glucocorticoids:* Women taking prednisone ≥30 mg/day for >1 month should consider therapy.
- *Men <40 Years:*
– *With Fragility Fracture:* Men who have a fragility fracture while on glucocorticoids should receive pharmacologic therapy.
– *With Accelerated Bone Loss:* Men without a fragility fracture but experiencing accelerated bone loss (≥4% per year) or a Z-score <-3.0 while on 7.5 mg prednisone equivalent for ≥3 months should also receive therapy.
– *High-Dose Glucocorticoids:* Men taking prednisone ≥30 mg/day for >1 month should consider therapy.
25% dose reduction…
40mg ka 25% is 10mg thats why 10mg is mentioned , if patient is on 40mg.
60mg ka 25% is 15mg, so 15mg should be reduced and new dose will be 45mg.
By the end of consolidation phase, 25% dose should be reduced. No matter patient is on how much steroid, us dose ka 25% nikal k wo reduce krna hai…
If on 40 then 25% would be 10,
If on 60 then 25% would be 15,
If on 80 then 25% would be 20
It will vary according to dose. 10mg is not fixed.
There are various protocols of tapering steroids…
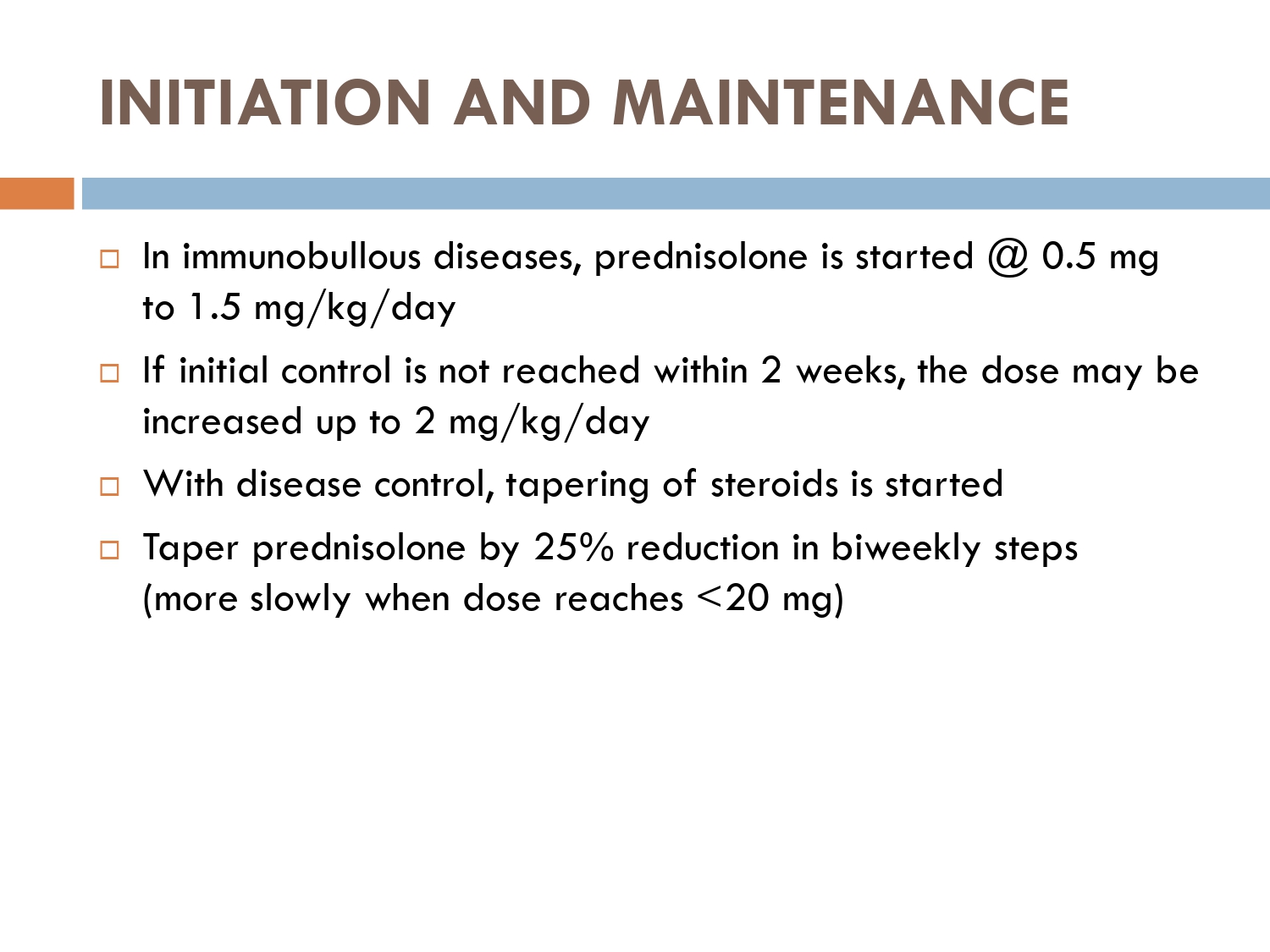
In the given scenario of immumobullous, we follow this european protocol.
Initial 25% dose reduction by the end of consolidation phase..
If patient reaches at less then 20mg its 5mg every month.. tapering becomes slow.




















🔰 Important
Steroids is the most imp drug which can be asked about in exam, n tht too in very much detail.
U must know each n everything about steroids bcoz from viva point of view ua entire viva may be based on this drug, n ultimately will decide ua fate too.


Mechanism of action
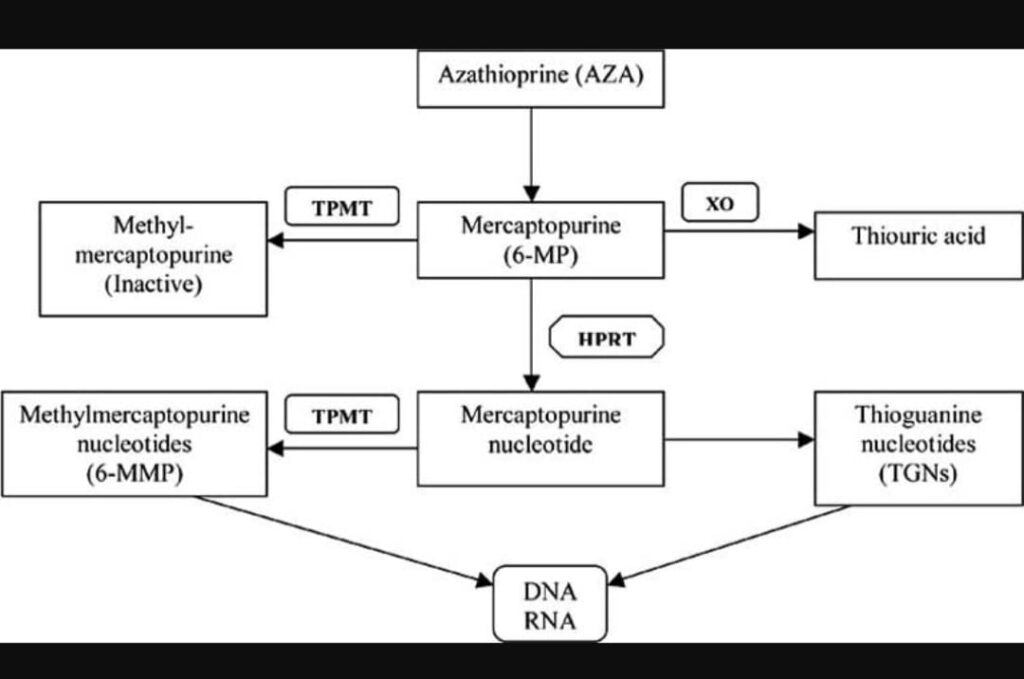
Azathioprine is prodrug that is rapidly transformed into 6-mercaptopurine.
By different enzymatic pathways it is converted into 6- thioguanine.
Incorporation of 6-Thiogunanine into DNA interfere with its replication and supression of purine synthesis.
So it has immunosuppressive, antiproliferative and anti inflammatory effect.
See the table for its metabolic pathway.
Adverse effects of azathioprine
Myelosupression : it is more likely with intermediate tpmt levels and increasing likely with low tpmt levels. Leukopenia is the most common haematological side effect, but anaemia, thrombocytopenia and pancytopenia can occur. It presents as infection bruising mouth ulcers and sore throat.
Carcinogenesis : lymphoma and scc
Infection : dissiminated herpes simplex, vzv hpv
Gastrointestinal : nausea vomiting diarrhea and pancreatitis
Hepatic : life threatening hepatitis due to oxidative stress. Nodular regenerative hyperplasia with resultant portal HTN presenting as thrombocytopenia and splenomegaly.
Hypersensitivity syndrome: in first month if treatment.
The syndrome is characterized by fever, malaise, arthralgia, myalgia, nausea, vomiting and diarrhoea, with occasional renal and liver dysfunction, pancreatitis, hypotension, cardiogenic shock and leucocytosis. There are clinical and histological features of a neutrophilic dermatosis and, although the eruption can be non‐specific in appearance, it may resemble Sweet syndrome, erythema nodosum, acute generalized exanthematous pustulosis or leukocytoclastic vasculitis. It settles within days of discontinuing AZA: rechallenge should not be undertaken for fear of causing a life‐threatening shock reaction.
Dosing regime of AZA according to TMPT levels
The empirical dose of AZA is 1-3 mg /kg with low starting dose of 0.5-1mg/kg.
Normal TPMT activity: 24.0-44.0 U/mL
So if
🩸TMPT > 19 the dose of AZA is 2-3mg/kg
🩸TMPT 13.7-19 the dose of AZA is 1-1.5mg/kg
🩸TMPT 5-14.7 dose of AZA is 0.5mg/kg
🩸TPMT <5 pmol/h/mg HB the AZA is Contraindicated…
allopurinol inhibits xanthine oxidase.
Xanthine oxidase converts 6 mercaptopurine into thiouric acid. When xanthine oxidase will be inhibited by allopurinol it will increase concentration of 6mercaptopurine and result in toxicity of azathioprine
If TPMT levels cant be done then azathioprine starting dose: is 0.5mg/kg/day with meal in order to avoid nausea
If pt develope allergic reaction to azathioprinein 3 to 4 days like flu like symptoms and increased temperature u have to stop azathioprine.
Monitor for cytopenias, if not detected then increase dose by 0.5mg/kg after 6-8weeks. If well tolerated further inc dose by another 0.5mg/kg after 4 weeks.
Do CBC and LFT weekly for 6 weeks then 2 weekly 6 weeks until dose is stable
Stable dose: CBC and LFT
Monthly for 3 months
Then every 3 monthly there after
Indications of azathioprine in Dermatology
*Pemphigus vulgaris
*Systemic lupus erythematous
*Dermatomyositis/polymyositis
*Use as steroid sparing agent in a treatment of bullous pemphigoid.
*Oral monotheraphy in the treatment of chronic Actinic dermatitis.
*Azathioprine is also used to treat a range of vasculitides including Wegeners granulomatosis,Behcets,HSP.
*Small studies has reported to show benefits in Pyoderma Gangrenosum ,Lichen planus , erythema Multiforme and pityriasis Rubra pilaris.
Advise of azathioprine use regarding conception and lactation.
Category D in pregnant females.
The prodrug and the active metabolites readily cross
the placenta.
The rate of congenital malformation is low (4.3%), but myelosuppression and immunosuppression are significant occurrences in neonates and breastfed infants(ref: Fitzpatrick)
Contraindicated in women who are pregnant or considering conception (unless benefit to the patient out weigh the risks)(Ref :Rooks 19.9)
If the patient is on azathioprine before pregnancy, this should be continued as there is no evidence of an increase in the rate of malformations.
In patients taking azathioprine no adverse events have been reported in breastfed infants exposed to the drug,
The majority of azathioprine is excreted within 4 h of ingestion, prompting the recommendation that feeds be given at least 4 h post maternal dose.
Azathioprine is compatible with pregnancy at a daily dose not exceeding 2mg/kg per day. It is compatible with breast feeding and paternal exposure..
MECHANISM OF ACTION OF POTASSIUM IODIDE.
It has anti inflammatory effects.inhibits neutrophils chemotaxis and inhibits release of pro inflammatory cytokines and reduces inflammation
DERMATOLOGICAL INDICATIONS:
Sporotrichosis
Erythema Nodosum
Subacute nodular migratory panniculitis
Sweet syndrome
Nodular vasculitis
Behchet’s disease
Pyoderma Gangrenosum
Erythema multiforme
🛑 Adverse effects of potassium iodide
🔆Wolff–Chaikoff effect:
Inhibition of organic binding of iodide in the normal thyroid gland by excess iodide. This is a protective mechanism, blocking thyroid hormone synthesis, and predisposing to temporary hypothyroidism.
Resolution usually occurs within several weeks, although thyroid replacement therapy may need to be considered.
🔆Jod–Basedow phenomenon:
There is a risk that the therapeutic administration of potassium iodide to a person with thyroid dysfunction lacking pituitary control, such as Graves disease or a multinodular goitre, may cause a significant exacerbation of hyperthyroidism.
🔆Potassium toxicity:
Rarely, potassium iodide may cause symptoms of hyperkalaemia, including fatigue, confusion, palpitations, muscle weakness and numbness and tingling of the extremities.
🔆Other adverse effects (iodism)
1) Sialadenitis (in particular parotid and submaxillary glands)
2) Hypersalivation
3) Metallic taste, halitosis, burning sensation in the mouth
4) Coryzal symptoms, headache
5) Gastric irritation, diarrhoea, nausea
6) Urticaria/angio‐oedema
7) Acneform pustulation
8) Iododerma
Use Cautions in following conditions
Thryoid and cardiac disorders
In States of hyperkalemia.
Active Tuberculosis
Can exacerbate conditions like
DH
Pustular psoriasis
Hypocomplementinic vasculitis.
Drug interactions.
Lithium
Antithyroid agents .
Potassium sparing diuretics (amiloride,spironolactone)
amiodarone
sulphonamide to cause hypothyroidism
ACE inhibitors to cause hyperkalaemia.
Pretreatment screening
1 Pregnancy test to rule out conception
2 serum T4, TSH, circulating thyroid antibody levels
3 baseline electrolytes esp potassium levels
4 serum urea levels
5 CXR and mountox skin test for latent T.B
6 baseline ECG and echo to rule out any preexisting cardiac disease
7 Serum potassium levels
*Monitoring of
POTASSIUM IODIDE*
🔹️TSH levels should be checked 1 month after initiation of therapy to exclude hypothyroidism..
🔹️Incase Abnormal it should be monitored thereafter..
Contraindications
Known hypersentivity
Pregnancy
Active TB
Hyperkalaemia
Urticarial vasculitis
Thyroid disease
Dec renal function
Cardiac disease
Pustular psoriasis

Indication of Glucantime
1.Cutaneous Leishmaniasis
2.Mucocutaneous Leishmaniasis
3.Visceral and postkalazar Leishmaniasis
4.Diffuse cutaneous leishmaniasis
Indications of systemic glucantime
- lesion ≥4 cm (plaque)
- Multiple lesions
- ‘delicate’ localisation in eyelids, lips, nose, ears or joints.
- lymphatic spread
- previous administration of topical antileishmanial therapy which failed
- Infecting Leishmania species itself that strongly influences the risk of developing ML.
- immunocompromised or uncontrolled diabetes
side effects of pentavalent antimony
🌟Local
injection site reaction
urticaria
pain sterile abcess
🌟Systemic
nausea
vominting
fever
myalgia
arthralgia
Cardiotoxicity ECG changes:
flat or inverted T waves
prolong Q-T interval
concave ST segment
Hepatotoxicity
Leucopenia and thrombocytopenia
Increased amylase levels and pancreatitis
Haemorrhagic nephritis
Dose of Sodium Stibogluconate :
Systemic
Sodium stibogluconate is usually administered by intravenous or intramuscular injection daily at a dose of 20 mg/kg/day (maximum 850 mg) for 21–28 days.
What ever wt is mentioned u can’t cross above 850 mg
Intralesional injections
Dose is 0.1 mL/cm2 (ie, 0.2–5 mL in up to 4–5 sites) repeated every 3–7 days until healing occurs.
Overdose is treated with dimercaprol given as deep IM injection
Dimercaprol is a dithiol that acts by forming a stable five-membered ring between its sulfhydryl groups and certain heavy metals, thereby neutralizing its toxicity and promoting its elimination.
IM Dosing
2.5 mg/kg QID x 2 days (days 1 & 2)
then bid for 1 day (day 3)
then qd (days 4-10)
It is available as 300 mg/vial for deep IM use (suspension in peanut oil).
Pretreatment investigations
Complete blood count — antimonials can cause transient thrombocytopenia and leucopenia.
Liver function tests — stop treatment if either ALT or AST is greater than 3–4 times the upper normal limit.
Serum amylase and lipase —stop treatment if amylase is more than 5 times the upper limit or lipase is more than 12 times the normal upper limit.
Renal function tests for serum urea and creatinine.
ECG — stop treatment if a T-wave inversion or prolonged QT interval occurs
🟩Repeat above investigations 10 days after treatment




Click Here to View the Low-Dose Oral Minoxidil for Alopecia: A Comprehensive Review


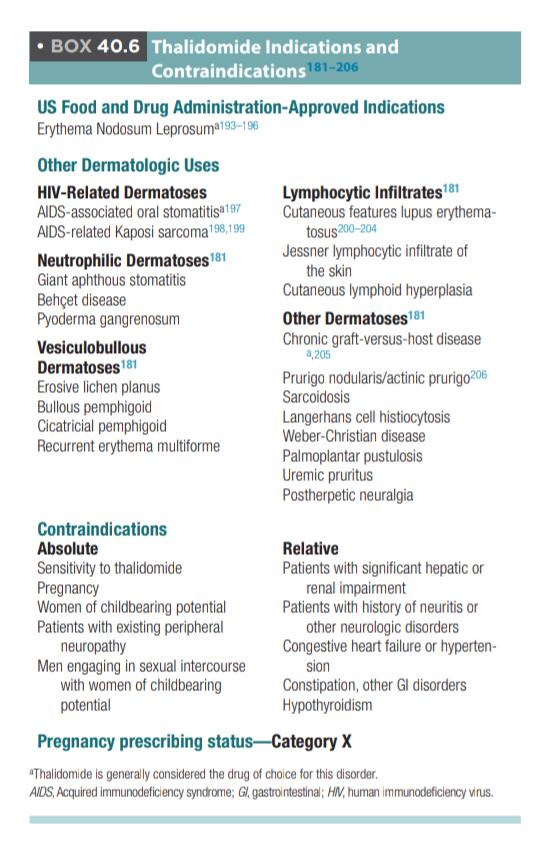
Indications
The main indications of Thalidomide are:-
Actinic prurigo
Nodular prurigo
Erythema nodosum leprosy,
Chronic cutaneous lupus./DLE
Other indications:
Erythema multiforme
Crohn disease
Systemic mastocytosis
Non- dermatological uses of thalidomide :
*Multiple myeloma
*Primary brain malignancies
*HIV- associated wasting syndrome
*Myelodysplastic syndrome
*Hematopoietic stem cell transplantation
*Hereditary hemorrhagic telangiectasia
Adverse effects
Teratogenic
Esp if pt is exposed
between 20 and 36 days after conception
Peripheral neuropathy
Mainly sensory
Venous and arterial thrombosis
Endocrine
hypothyroidism
Hypo‐ and hyperglycaemia, hypocalcaemia
Cbc
Anaemia
neutropenia
Lymphopenia thrombocytopenia Erythroderma, Stevens–Johnson syndrome/toxic epidermal necrolysis,Hypersensitivity reaction
Drowsiness, dizziness, depression, confusion, anxiety, agitation
Intestinal obstruction and perforation,Dry mouth, constipation, nausea*
Bradycardia, peripheral oedema, dyspnoea, cardiac failure*
Decreased libido*
Xerosis,pruritus*
Monitoring of Thalidomide
Before starting treatment👇🏻
Neurological examination to check any signs of peripheral neuropathy
°Baseline investigations including CBC, LFT, pregnancy test
After initiating treatment👇🏻
°Monthly neurological examination for first 3 months and then 6 monthly
°Monthly pregnancy test and 4 week after cessation f treatment
°CBC, LFT monthly until the dose of thalidomide is stable, then every 2–3 months.
°Electrophysiological testing every 6 months to detect subclinical neuropathy
Thyroid function test every 3 months for hypothyroidism
🔸 Regarding advice : advice pt to take drug at night time as it causes drowsiness.
And seek medical attention if he develops the sign and symptoms of calf pain, chest pain, shortness of breath for thromboembolism.
Also explain pt that it has slow onset of action and benefit may not appear until 2-3 months of treatment.
❇️ MECHANISM OF ACTION
Cyclophosphamide is an alkylating agent of the nitrogen mustard type. An activated form of cyclophosphamide, ‘phosphoramide mustard’ alkylates, or binds IRREVERSIBLY to DNA. Its cytotoxic effect is mainly due to cross-linking of strands of DNA and RNA, inhibits protein synthesis leading to inhibition of cell division and replication.
It also decreases T cell mediated immunity and B cell proliferation.
❇️ DRUG INTERACTIONS
🟠Allopurinol
🟠Amiodarone
🟠Chloramphenicol
🟠Ciprofloxacin
🟠Corticosteroids
🟠Digoxin
🟠Grapefruit juice
🟠Hydrochlorothiazide
🟠Indomethacin
🟠Phenytoin
🟠Phenobarbital
🟠Succinylcholine
🟠Warfarin
Parischia regime/ DCP regime
◼️Day 1-3 :100 MG dexamethasone in 500 mL of 5% glucose as an IV infusion over two hours.
◼️Day 2: 500 mg cyclophosphamide added to the dexamethasone IV infusion
◼️day:4-28 cyclophosphamide 50mg daily with conventional daily doses of oral corticosteroids.
This cycle is repeated every 28 days until Clinical remission is achieved and oral corticosteroid with drawn(phase 1 )typically 3 to 4 months.
then DCP continues in 28 days cycle with 50mg oral cyclophosphamide in between the pulse for the nine months (phase 2),
and then oral cyclophosphamide 50mg daily continued for another nine months (phase 3) treatment then withdrawn (Phase4)and patient follow-up foot up to 10 years ..
⚫ Modifications to this regimen include
IV methylprednisolone to 250-1000mg (3 to 5 days )instead of dexamethasone
Another approach is to combine conventional daily oral corticosteroid with monthly IV cyclophosphamide pulses 15 mg/kg .
Monitoring of cyclophosphamide
For monitoring..check cbc and urinalysis—weekly then fortnightly
Then every 2-3 months
Lfts- monthly..
Mesna should be given along with iV cyclophosphamide for haemorrhagic cystitis.
Patient should be counselled for haematuria .
With iv infusion B.P monitoring and urine output is maintained




Mechanism of action of Retinoids:
Enter nucleus
Bind to specific receptors on DNA..
Synthesis of mRNA and target protein..
Proteins affect differentiation,proliferation and immune regulation
What are rexinoids?
rexinoid are vitamin A compounds which act by engaged by nuclear retinoid X receptors, RXRs help prevention cancer
Baxoreten
Systemic retinoids
Pre treatment screening
For women of child bearinh age pregnancy should be excluded
Pregnancy should be avoided for duration of therapy and for an appropiate time thereaftwr(1 month for isotretinoin,alitretinoin,baxarotene and 2 yrs after acitretin).
IPLEDGE(ISOTRETINOIN PREGNANCY PREVENTION PLAN) which involves pt education about retinoid s/e and contraception,written informed consent,the employment of ideally 2 adequate forms of contraception established at least 1 month prior to initiation of therapy and a negative urinary or serum pregnancy test.
Amenorrhoic women should have pragnancy test 14 days after last act of coitus.
Other pretreatment lab tests:
CBC
LFTs
RFTs
Full fasting lipid profile
Thyroid function test(for alitretinoin and baxarotene)
Adverse effects
Dermatological(cutaneous and mucosal)
Skin – dryness , palmoplantar peeling, exfoliative dermatitis,photo sensitivity, facial erythema, pruritis,impetignization,asteatotic eczema and fragility ,sticky skin( treated with emolients) pyogenic granuloma like lesions
Acne fulminans -v. Rare
Oral mucosa-chelitis
Nasal mucosa- dryness,epistaxis
Ocular- dry eyes, blepharoconjuctivitis,
Hair- telogen effluvium, hair thinning
Nails- fragile nails, onycholysis paronychia
Systemic
Teratogenicity-retinoid embryopathy( in first trimester ) include
craniofacial deformities( cleft palate)
CNS( hydrocephalus,microcephaly,cerebral malformation)
Heart( TOF,transposition of great vessel,septal defect) thymus and parathyroid abnormality, ocular, renal, pulmonary, skeletal qnd limb defects
Psychiatric- depression,suicidal ideation, personality disorder
Ocular-delayed dark adaptation(retinal toxicity)photophobia,blurred vision ,dry eyes, blepharoconjunctivitis, corneal opacities
GI-Nausea diarrhea, abdominal pain, increase liver enzymes and bilirubin
Neuro-transient headache,
Pseudotumor cerberi( more when concomitant used with oral tetracycline)
MSK-hyperostosis with premature epiphyseal closure, calcification of tendons and ligaments,decrease bone mineral density,myalgia, DISH-diffuse interstitial skeletal hyperostosis
Hematologic-leucopenia and agranulocytosis, thrombocytopenia , neutropenia, anemia
Metabolic-dyslipidemia, hypertriglyceridemia,hypercholestrolemia
(Reversible)
Endocrine- central hypothyroidism (bexarotene and alitretinoin)
How to prevent psychitric complications?
depression may be a rare, idiosyncratic and unpredictable adverse effect of isotretinoin therapy. To prevent It ⚠️advise patients and members of their families to be
watchful for signs and symptoms of depression and to make specific
enquiries relating to mood and suicidal thoughts. ⚠️
introducing isotretinoin at a low dose (0.5 mg/kg body
weight per day) before gradually increasing the dose of isotretinoin
after 2 months if required may lessen the risk of this.
Source: rooks 19.38
Dose of Isotretinoin
.Initial dose is 0.3-0.5 mg/kg/day Sustained dose is 0.5-1 mg/kg/day
Topical gel is 0.05%.. Topical cream is 0.5 mg
Systemic Isotretinoin strenght in capsule is 5mg,10mg,20mg and 40 mg.
In pakistan it is available by the name of
Cap Arynoin Isozam cap,Maxinoin cap,Atractin .
Acitretin
Dose Initial 0.2-0.5 mg/kg/day and sustained dose is 0.3-0.8 mg/kg/day..
It comes in 10mg,25mg. Brand name Neotret cap,Tigasor capsule,Acetin cap.
Bexarotene
dose is 4-8 mg/kg/day. Topical gel in 1%
Alitretinoin
indication is in Hand eczema when topical treatment fails and in HIV KAPOSI sarcoma.
contraindications of retinoids
Absolute
*Pregnancy &
*Lactation (cat X drug)
*Hypersensitivity to retinoids/soya/peanut
*Pt who are receiving tetracyclin
relative
Renal insufficiency
Hepatic insufficiency
Severe hyperlipidemia
Contraceptions duration required after stopping retinoids
1month for
Isotretinoin , alitretinoin & baxarotene
2 years for acitretin
Cautions
Exposure to intense sun light sould be avoided
Wax depilation should be discouraged for 6 months for fear of epidermal stripping.
dermabrassion on laser resurfacing shoild be post poned for 6 months due to risk of hypertrophic scarring and dyspigmentation.
Indications
Isotretinoin:severe acne
Alitretinoin:chronic hand eczema
Acitretin:severe psoriasis
Baxoretene:cutaneous TCell lymphoma..
Indications
Isotretinoin:severe acne
Alitretinoin:chronic hand eczema
Acitretin:severe psoriasis
Baxoretene:cutaneous TCell lymphoma..
Pre treatment monitoring of retinoids*
monthly clinical evaluations and blood test are done with start of retinoids,where Lfts and Flps are done regularl with occasional Renal function tests and cbc for 3-6 monthly,then 3 monthly reviews of blood tests.
Thyroid functions are assessed if pt is on Alitretinoin or bexarotene
Serum and urine Pregnancy test monthly for women of child bearing age and again 5 weeks after drug is stopped.
Specific enquiry should be made about adverse effects related to mood and vision
Routine monitoring for skeletal toxicity or osteoporosis is not required for Asymptomatic pts but if pt reports restricted mobility and bone pain then relevant radiological examination should be done.
🔮 Mechanism of action
💊Retinoid enters the cell , interact with nuclear receptor involved in epithelial cell growth and differentiation
💊Reverses the thickening of st corneum
💊Reverses the abnormal desquamation of keratinocytes
💊 It normalizes follicular keratinization
💊New comodones formation is inhibited
💊Comodons are extruded
Types of retinoids
Retinoids are classified into four generations:
🟩- 1st generation (non-aromatic retinoids):
retinol, retinal, tretinoin (retinoic acid), isotretinoin, and alitretinoin
🟩- 2nd generation (mono-aromatic retinoids):
etretinate (withdrawn from the market) and its metabolite acitretin
🟩- 3rd generation (poly-aromatic retinoids):
adapalene, bexarotene, and tazarotene
🟩- 4th generation retinoid:
Trifarotene.
Adverse effects of topical retinoids
Skin discoloration.
Photosensitivity to UV light.
Initial acne flare-up.
Eczema flare-up.
Swelling of the skin.
Blistering and stinging.
Pyogenic granuloma,
onycholysis
telogen effluvium
Dosing schedule of topical retinoids
💦TOPICAL TRETINOIN
⚡ACNE
Topical administration of includes applying a thin layer once daily, before bedtime, to the skin where lesions are present. Patients need to keep the medication away from eyes, mouth, nasal creases, and mucous membranes.
Dosages vary amongst different brands. A commonly used topical tretinoin consisting of 0.1%, 0.08%, and 0.04% dosages should be applied once daily, during the evening, to the skin where acne lesions appear to be present, with enough to cover the entire affected surface in a thin layer.
⚡Usual Adult Dose for Dermatoheliosis
Recommended dose: Apply a pea-sized amount to the entire affected area once a day at bedtime
Duration of therapy: 48 weeks (0.05% cream/emollient cream) and 52 weeks (0.02% cream)
Comments:
-Approximately 20 to 30 minutes prior to application, patients should wash their faces with a mild soap and pat the skin dry.
-Warmth and/or stinging may occur after application.
-Improvement in fine wrinkling may not be observed for up to 6 months.
Drug interactions of retinoids
🏀tetracyclines increase the risk of benign intracranial hypertension
🏀alcohol increases half life of acitretin
🏀dose of simvastatin and anticoagulants is reduced by acetretin
🏀dose of carbamazepine is reduced by isotretinoin
🏀 ketoconazole increases conc of alitretinoin
🏀metho toxicity with acitretin
🏀concomitent vit A leads to hypervitaminosis


mechanism of action
Ans:☘️ It is a human igG1 monoclonal antibody that inhibits the member of cytokine family that is interleukin 17A which is mainly produced by inflammatory T helper 17 cells.
☘️ IL 17 is increased in serum of psoriasis patients and synovial fluid of patients with psoriatic arthritis and promotes inflammation when binds to IL 17 receptor which is expressed by various types of cells including keratinocytes in skin.
How to prepare an ampule of secukinumab for administration?
💉Secukinumab comes formulated as lyophilized powder of 150 mg in a vial,product reconstitution is performed with 10 ml sterile distilled water, followed by gentle stirring to dissolve the powder, and kept aside for 10 mins. Finally, the solution must be inspected to be free of residual particles or discoloration, and administration must follow promptly.
💉prefilled syringe, or a sensory-ready pen that requires storage at a cool temperature (2 to 8 degrees C , Before administration, the pen or prefilled syringe must be kept aside for 20 to 30 mins until it reaches room temperature
💥FDA-approved indications for Seckukinumab IV( Cosentyx,Novartis)
Moderate to severe psoriasis
hypertrophic palmoplantar
Generalized Pustular psoriasis
Psoriatic Arthritis
Ankylosing spondilitis
Juvenile psoriatic arthritis aged 2 years or older
⭕OFF LABEL USES
1.Rheumatoid arthirits
2.SLE
3 Familial Mediterranian Fever
4 .TRAPS
🔆Source🔆
https://www.ncbi.nlm.nih.gov/books/NBK537091/
Dosage and route of administration
Route of administration
Subcutaneous
Schedule
🔅PLAQUE PSORIASIS: 300mg at 0,1,2,3,4 weeks then 4 weekly.
🔅PSORIATIC ARTHRITIS: 150 mg at 0,1,2,3,4 weeks then 4 weekly.
Brand name,and pricing information of Secukinumab
A: Brand name: Cosentyx ( by Novartis)
▪️ 75mg/ml, 150mg/ml and 300 mg/2ml prefilled syringe
Fraizeron 150mg vial(by novartis)
Price : 1 injection 150mg prefilled syringe : around 60,000
” ADVERSE EFFECTS OF SECUKINUMAB”
-The most common adverse drug reactions with secukinumab are nasopharyngitis, upper respiratory tract infection, and diarrhea(>1%).
-Other adverse effects include:
-Inflammatory bowel disease and exacerbation of Crohns disease
-Urticaria and anaphylaxis
Angioedema, chest tightness
-Headache
-Pruritus
-Hypertension
-Arthralgia and back pain
-Cough
-Rhinorrhea
-Infections
-Pyoderma
-Malignancies
-Nonmelanoma skin cancer (basal cell carcinoma, squamous cell carcinoma)
-Melanoma
-Bladder cancer
-Thyroid cancer
-Neutropenia
-Injection site reactions
-Mucocutaneous candidiasis
CONTRAINDICATIONS OF SECUKINUMAB”
🟩Absolute contraindications
🔅Active infections, more importantly, latent or active tuberculosis, hepatitis B, C, and HIV
🔅 hypersensitivity to secukinumab or latex.
🟩Relative contraindications include:
-PUVA sessions
-Premalignant conditions
-Demyelinating disease
-Optic neuritis
-Multiple sclerosis
-Children
-Pregnancy and lactation
-Congestive heart failure
-Fever
-Jaundice or marked liver enzyme elevations
-Inflammatory bowel disease
-Concurrent use of live vaccines with secukinumab
🔮 Q: Can Secukinumab be used in patients with a history of malignancy?
💎 A: The safety of Secukinumab in patients with a history of malignancy has not been established. Caution should be exercised when considering treatment with Secukinumab in patients with a history of malignancy, and the potential risks and benefits should be carefully weighed on a case-by-case basis. Close monitoring for signs of malignancy recurrence or progression is recommended during treatment with Secukinumab.
🔮 Q: How does interleukin-17A contribute to the pathogenesis of psoriasis?
💎 A: Interleukin-17A (IL-17A) is a key cytokine involved in the pathogenesis of psoriasis. It promotes the activation and recruitment of various immune cells, including neutrophils, T cells, and dendritic cells, leading to inflammation and keratinocyte hyperproliferation. IL-17A also induces the production of other pro-inflammatory cytokines and chemokines, exacerbating the inflammatory cascade and contributing to the development of psoriatic plaques.
🔮 Q: Can Secukinumab be used in patients with comorbidities such as diabetes or hypertension?
💎 A: Secukinumab can generally be used in patients with comorbidities such as diabetes or hypertension. However, caution should be exercised in patients with uncontrolled comorbidities, as there may be an increased risk of complications. Close monitoring and coordination with other healthcare providers may be necessary to ensure optimal management of comorbid conditions during treatment with Secukinumab.
🔮 Q: What is the efficacy of Secukinumab in patients with nail psoriasis?
💎 A: Secukinumab has demonstrated efficacy in the treatment of nail psoriasis, characterized by improvements in nail bed and nail matrix psoriasis severity scores. Clinical trials have shown that treatment with Secukinumab leads to significant improvements in nail psoriasis symptoms, including nail pitting, onycholysis, and subungual hyperkeratosis.
🔮 Q: Are there any special considerations for the use of Secukinumab in elderly patients?
💎 A: Elderly patients may be more susceptible to certain adverse effects of Secukinumab, such as infections. Therefore, caution should be exercised when initiating treatment in this population, and close monitoring for signs of infection is recommended. Additionally, elderly patients may have age-related changes in renal or hepatic function that could affect drug metabolism and clearance, so dose adjustments may be necessary in some cases.
🔮 Q: How does Secukinumab compare to other biologic agents in the treatment of psoriasis?
💎 A: Secukinumab has demonstrated efficacy in the treatment of psoriasis, particularly in patients who have not responded adequately to other systemic therapies. It has been shown to have comparable or superior efficacy to other biologic agents such as etanercept and ustekinumab in clinical trials.
🔮 Q: Can Secukinumab be used in pregnant women?
💎 A: The use of Secukinumab in pregnant women is not recommended unless the potential benefits outweigh the risks. Pregnant women should consult their healthcare provider before using Secukinumab.




Mechanism of action
Apremilast is a PDE4 inhibitor which affects production of inflamatory cytokines from T lymphocytes.
PDE4 is the predominant phosphodiesterase in-
volved in the control of activity in inflammatory
cells
It is also expressed in keratinocytes.
Through inhibition of PDE4, apremilast causes an
elevation of cyclic adenosine monophosphate, a
naturally occurring intracellular secondary messen-
ger that functions as a modulator of inflammatory
responses,thereby decreasing production of
proinflammatory mediators such as
TNF
Interleukin (IL)-23, and Interferon gamma
and increasing production of anti-
inflammatory mediators, such as IL-10
💥 Indications of Apremilast
👉🏽Psoriatic Arthritis
👉🏽Plaque Psoriasis
👉🏽Oral ulcers with behcet disease
It is FDA approved for chronic plaque psoriasis and psoriatic arthritis esp dactylitis and enthesitis,esp in those patients who are candidates for phototherapy or systemic therapy …there is marked improvement in DLQI after it’s use.
👉🏼 Atopic eczema
👉🏼 Hydradenitis suppurativa
Side effects
👉🏻Generally well tolerated except mild nausea and dirrhea
👉🏻 >10%
Diarrhea
Nausea
👉🏻 1-10%
Upper respiratory tract infection and other infections
Tooth abscess
Folliculitis
Headache/migraine
Fatigue n depression
Dyspepsia
Vomiting
Decreased appetite
Insomnia
Back pain
Arthralgia
Malignancy
👉🏻Other side effects
Hypersenstivity
Mild allergic reactions
weight loss
Pre treatment screening.
Blood CP
LFTs
RFTs
Pregnancy test
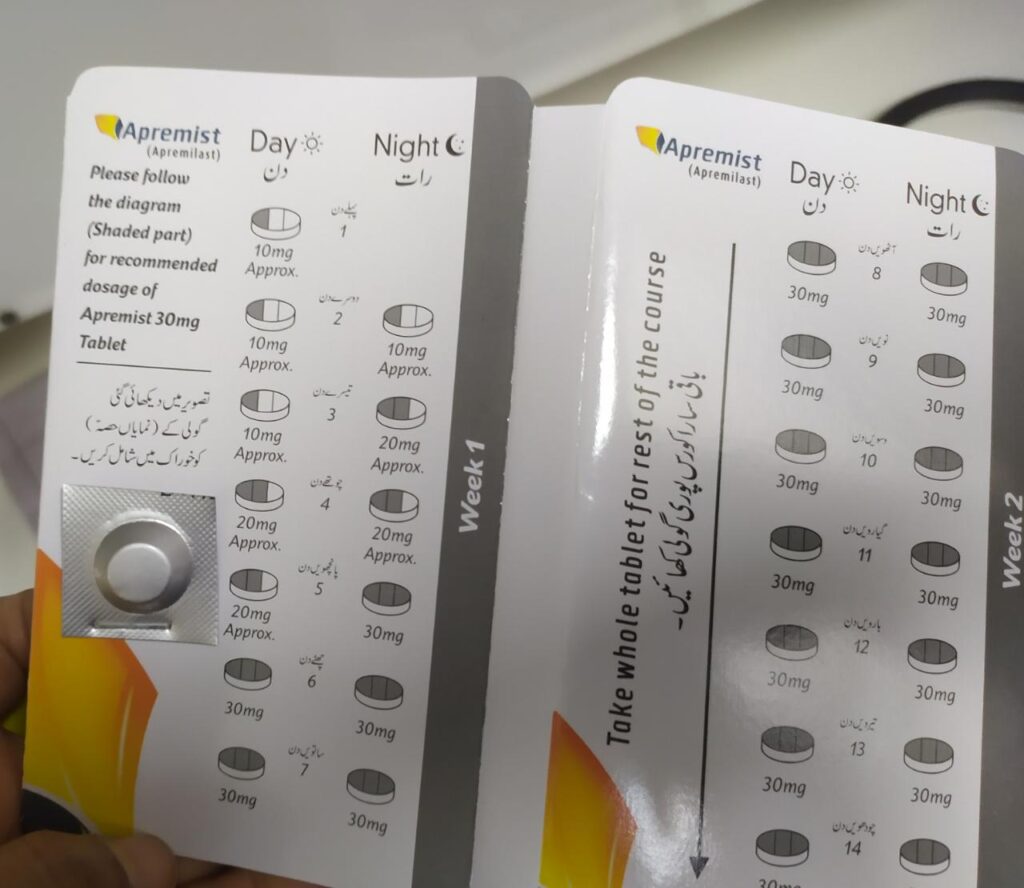
Dosing of apremilast
Oral Administration.
To reduce risk of GI symptoms associated with initial therapy, titrate slowly to recommended dose (30 mg PO BID) according to the dosage
In Pakistan available by the name of Apremist
Pack of 56 tabs in 3560 rs
63rs/tab
Kuniz Tablet
By Tabros pharma
Pack rs 2000
Contains Day to day dosage pills
30/20/10
Very nice drug and Cheap









🟩Antiinflammatory effect of antimalarials is due to dec in prostaglandin levels
SIDE EFFECTS
♦️What are its oculotoxicity?
Corneal deposits of HCQ
Asymptomatic and may present as visual haloes.
Reversible on stopping the drug.
Retinopathy
Important vision‐threatening side effect.
Cause typical ‘bull’s eye’ maculopathy (an annular zone of depigmentation in the pigment retinal epithelium surrounding the fovea ) , changes become visible on fundoscopy. Patient complaints of difficulty with reading. Irreversible on stopping the drug.
Ocular toxicity:retinopathy is much less with hydroxychloroquine than with
chloroquine.
Baseline eye examination should be done.
Then yearly in pts wd at risk of development oculotoxicity
Like if pt z on more than 5mg/kg dose
Renal liver failure
If no risk factors then 5 yearly
Risk factors of retinopathy:
Daily dose : >5mg/kg for HCQ
>2.3mg/kg for chloroquine
Duration of use : >5 years
Cummulative dose:
1000g for HCQ
Renal impairement
Concomitant tamoxifen use
Pre-existing retinal and macular disease
♦️In which patients should it be used cautiously?
- Lactation & pregnancy
- Neuromuscular disease
- Psychotic conditions
- G6PD deficiency
- PCT
- patients with severe blood dyscrasias
- hepatic disorder
Skin reactions:
1.blue–gray to black hyperpigmentation on shins.
2.Diffuse yellowish discoloration with mepacrine
3.Reversible bleaching of the hair roots
(achromotrichia)
4.Exanthem, ranging from urticaria to lichenoid reactions to exfoliative
erythroderma
Gastrointestinal issues: Nausea, vomiting, stomach pain, and diarrhea.
Neurological effects: Headache, dizziness, and in some rare instances, seizures.
Cardiotoxicity
conduction abnormalities occur rarely
Dose of hcq 200-400mg daily but not exceeding 6.5mg/kg/day
🎯Hcq use more than 5mg/kg requires an annual ophthalmological work up as per American academy of ophthalmology
Dose of chloroquine: 2.5mg/kg/day
But low dose chloroquine 125mg or 250mg or hydroxychloroquine 100mg twice a week is t/m of choice in porphyria cutanea tarda bcz daily dose can cause dangerous acute hepatitis.
*Brand available* hydroxychloroquine: HCQ 200 (getz pharma)
Price: Rs 270/ 30 tablets
PLAQUIN-H (macter international) price: rs 270/ 10 tables
what are risk factors to rule out before giving hcq ?
💧Ask about any visual impairment : correctable or not
💧Can read small print size N6 or N8
💧ask about cigeratte smoking if yes counsel to stop
💧Any liver and renal impairment
💧Drug history esp.if antiepileptics Inc chances of fits
If taking cyclosporin Dec it’s dose
If anti-diabetic bcoz Inc chances of hypoglycemia
Pre- Rx monitoring
- CBC
- LFTs
- G6PD def
- Porphyria screening
- Visual assessment/ near vision test.
- Counselling regarding visual damage with written consent
While on Rx.
- CBC & LFTs monthly for first 3months then 4-6monthly while on Rx
- Visual symptoms – any visual impairment not corrected with glasses , should be assessed by optometrist.
If pt can read small print size, cont HCQ.
If visual error is correctable then continue Rx
If not correctable > ophthalmological assessment.
Ideally vision should be assessed before starting the Rx and then annually after 5 yrs, unless there are risk factors – in that case annual assessment should be done.
While on Rx and being assessed for oculotoxicity – if there are corneal deposits, no need to stop the drug.
But Bull’s eye maculopathy on fundoscope – indication to stop the drug. Although it’s progressive and stopping the drug has no effect on it.
From opthalamic point of view, investigations which are important
Visual acuity
Perimetery
Occulo retinogram
Occulo retinogram
Optical coherence tomography


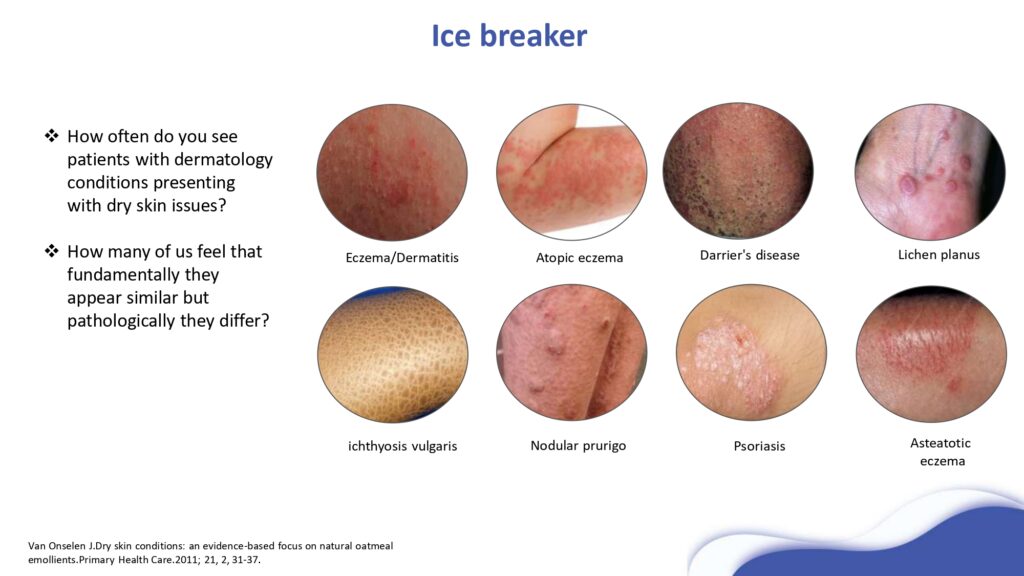













mechanism of action
🔅Antimitotic
🔅Anti inflammatory
🔅Inhibits Neutrophils degranulation,chemotaxis and phagocytosis with preferential accumulation in Neutrophils where concentration exceeds 16 fold of plasma concentration, reason why it is so effectively used in Neutrophilc dermatosis
Dermatological Indications of COLCHICINE
🟩FDA approved:
■Familial Mediterranean fever
■Acute flares of gout
🟩Other indications:
🔅Neutrophilic dermatosis
■ Behcet disease
■ Sweet syndrome
■ Pyoderma gangrenosum
■ Recurrent aphthous ulcers
■ Subcorneal pustular dermatosis
🔅Immunobullous dermatosis
■ Dermatitis Herpetiformis
■ Linear IgA disease
■ Epidermolysis bullosa acquista
🔅Papulosqamous disorders
■ Psoriasis
■ Palmoplantar pustulosis
🔅CTDs
■ Dermatomyositis
■ Scleroderma
🔅Panniculitis
■ Erythema nodosum
■ ENL
🔅vasculitis
■ Leukoocytoclastic vasculitis
■ Urticarial vasculitis
■ Hidradenitis suppurativa
Adverse effects
Cutaneous:
urticaria
sjs,TEN
alopecia universalis
pct
Purpura
Acute
Nausea
Vomiting
Cramping
Abdominal pain
Fatigue
Headache
Sore throat
Hypersensitivity
Rhabdomyolysis
DIC
Multiorgan failure
Chronic
Myelosuppression
Leukopenia
Thrombocytopenia
Granulocytopenia
Pancytopenia
Aplastic anemia
Severe diarrhea
Myopathy
Neuropathy
Hepatotoxicity
Nephrotoxicity
Multiple organ failure
Reversible azoospermia
Pre treatment investigations and monitoring
FBC
Rfts
Lfts
Urinanalysis
Pregnancy test.
Monitoring👇🏻
Fbc, rfts, lfts and urine analysis monthly for several months then 3 monthly
Ref rooks
Primary candidate:
Drug:colchicine
Brand name
Colchicine
Dose:0.5mg tablet
Price:169/- for 100 tablets
So each tablet of 2/- almost
Contraindication
Known hypersensitivity
Blood dyscrasias
-Patients with serious gastrointestinal, kidney, liver or heart disorders
Patients taking statins(cholesterol-lowering medications)
Pregnant women
Old age
Pregnancy category C drug
Colchicine toxicity
Divided into three stages.
1: Stage one; initial symptoms of burning of mouth, diarrhea, nausea, and vomiting within few hours.
It cause severe dehydration This stage typically lasts anywhere from 1-12 hours.
2: Stage two; usually lingers for 1-7 days
bone marrow suppression, multi-organ failure, kidney failure, altered mental status, and heart attack.
3: stage three; includes hair loss and rebound elevated blood count that will last 1-2 weeks for those individuals that survive stage two.
🎯 Drug–drug interactions
Drugs that inhibit the CYP3A4 and P‐glycoprotein systems may
increase colchicine levels and toxicity:
they include
🍒ciclosporin,
🍒erythromycin,
🍒clarithromycin,
🍒ketoconazole,
🍒itraconazole,
🍒antiviral
drugs and
🍒verapamil,
and grapefruit juice has a similar action
⚡ Co‐administration with statins may increase the risk of
myopathy
Colchicine cannot be removed by dialysis or exchange transfusion
Vomiting,diarrhea and abdominal pain respond to dosage adjustment if failed then stop the drug.
Bone marrow suppression can be corrected by giving granulocyte colony stimulating Factor .
Myopathy recover on drug withdrawal.




TNF alpha inhibitors:
1)fusion protein = Etanercept
2)fully human monoclonal antibody = adalimumab, golimumab
3)humanized monoclonal antibody =Certolizumab
4)Chimaeric antibody =infliximab
Prerequisites before starting TNF alpha inhibitors
CBC
screening for hepatitis B and C , tuberculosis and HIV
ELISA for hep B and C and IGRA testing, CXR and quantiferon gold test for TB
HIV by PCR
Give test dose of infliximab and check for any hypersensitivity reactions and if any then give prior to administration antihistamines prednisolone and acetinophen etc…
Side effects.
Bacterial infections especially upper respiratory tract.
Rare opportunist infections .
Histoplasmosis
Listeriosis
Cryptococcosis
Candida
Neurological.
Peripheral demyelinating disease especially MS .
Heart disease.
Should be avoided in severe hear disease like NYH Association class 3 and 4.
Drug induced lupus like syndrome
Hepatic disease may derange LFTs
Lymphoma
Increase Risk of melanoma /skin cancer .
Haematological side effects like aplastic anemia ,thrombocytopenia
Pregnancy and lactation safety
Adalimumab, Etanercept, Infliximab all are category B and are also secreted in breat milk
Use during pregnancy and lactation should be avoided wherever possible
Certolizumab lacks Fc portion, doesnt cross placenta and any effect on baby’s immune system is minimal
♦️Infliximab:
🔹️Route:Intravenous
🔹️Dosing:
♣️5mg/kg at week 0,2,6
and then 8 weekly
🔹️Trade name:
♣️Remsima 100mg
By atco labs
Price Rs.33000
♣️Remicade 100mg
By Bayer Pakistan
Price Rs.46000
Adalimumab
HUMIRA 40mg/0.4ml injection
SC
80 mg x Stat
40 mg x next week
40 mg x q2week
(price in Pakistan is Rs. 37000 )
If a person is on tnf antagonists and is planning to go under major surgery, discontinue this drug prior to surgery
- 2 weeks for Etanercept
- 6-8 weeks for adalimumab
- 4-6 weeks for Infliximab
Etanercept does not produce neutralising antibodies
Indications
Licensed uses👇🏻
Psoriasis
Psoriatic arthritis
RA
Off label uses👇🏻
PG
Sweet syndrome
Hidradenitis suppurativa
IBD
BEHCET
Resistant sarcoidosis

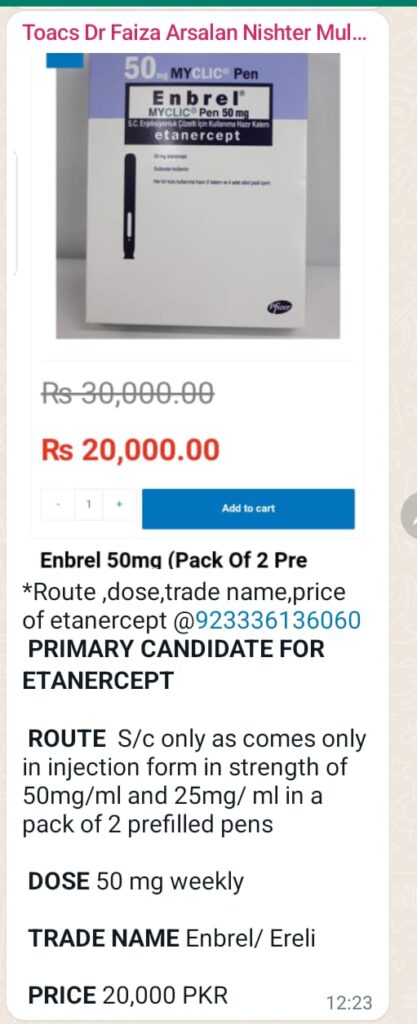





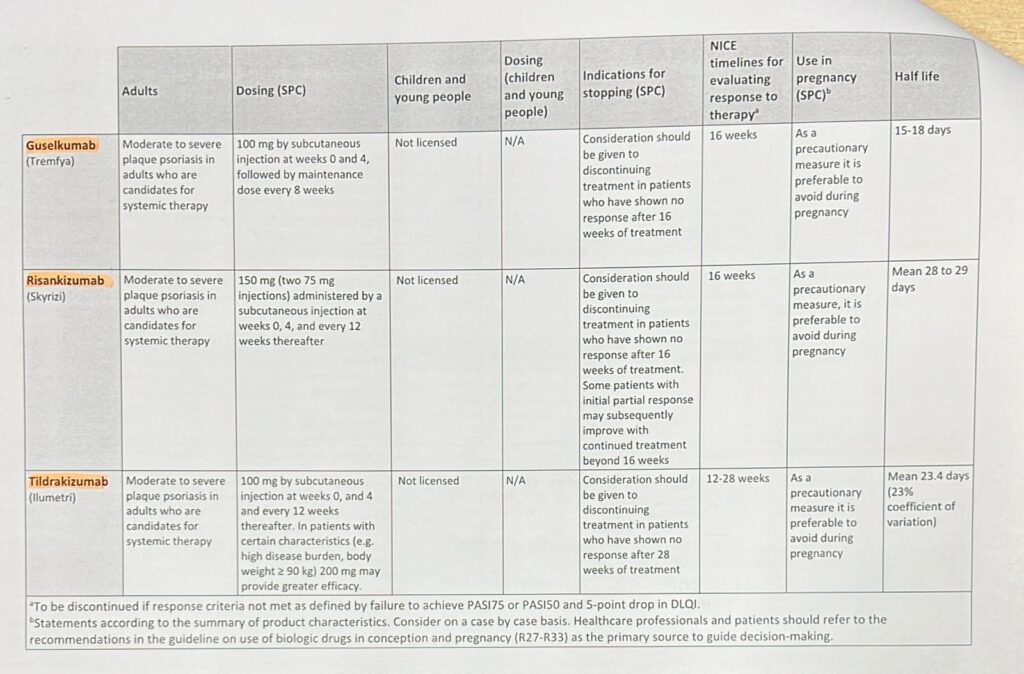

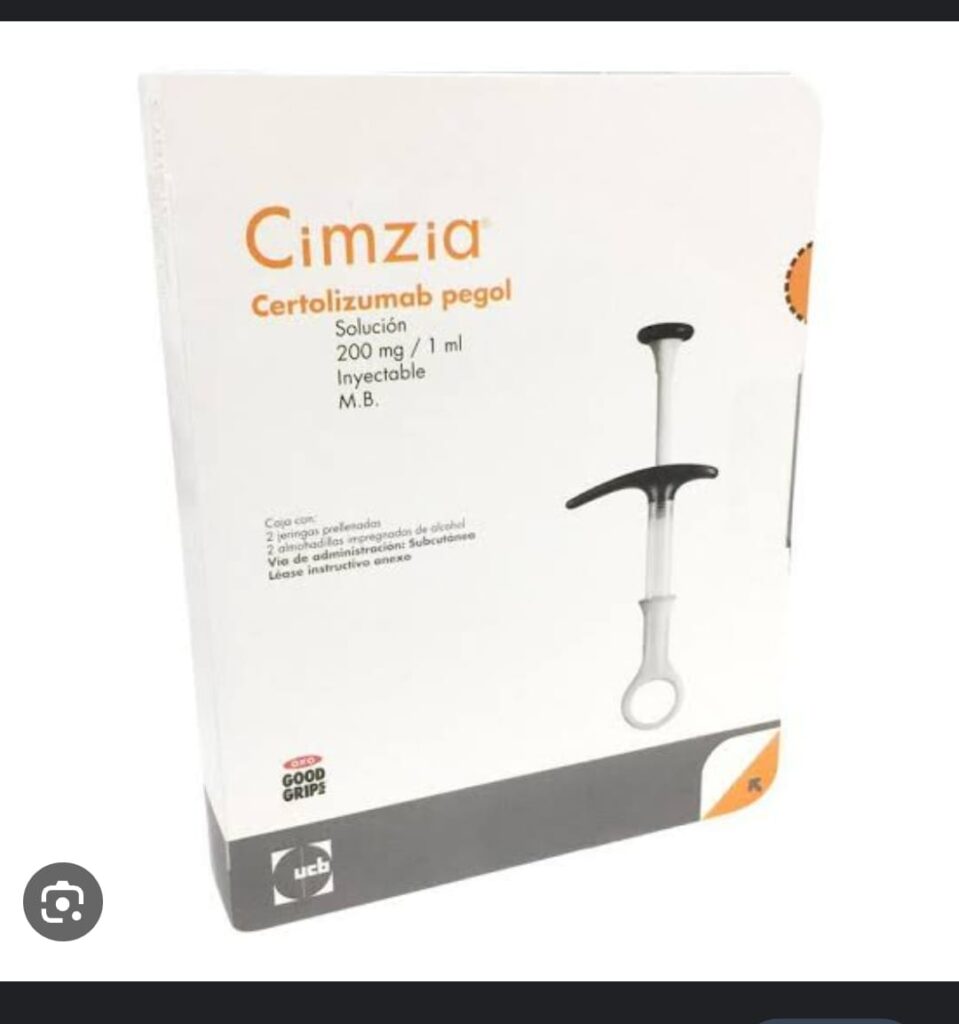

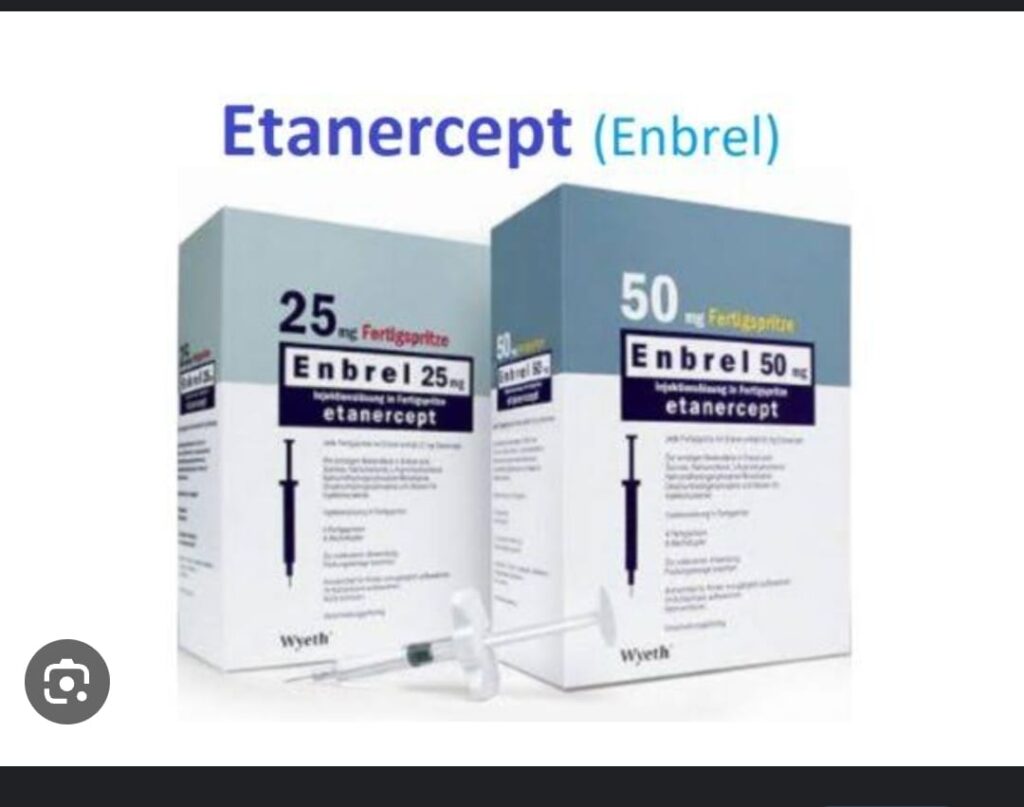



⭕ Indicataions of MMF
1 Immunbullous diseases (pemphigus, bullous pemphigoid)
2.Vasculitis
3..Psoriasis
4 Lichen planopilaris
5 lichen planus
6 Atopic eczema
7.pyoderma gangrenosum
8.Sarcoidosis
9.CTD: SLE, Dermatomyositis
- Morphea
🌀Non dermatological uses
1 Prophylaxis of transplant rejection e.g
♦️Renal transplant rejection
♦️Cardiac transplant
♦️liver transplant rejection
2…For symptoms of carcinoid tumors
Indications of mmf in sle..
1) moderate to severe disease activity
BILAG A or B
SLEDAi more then 6
Systemic involvement starts platelets less then 50000 /lit
Rash involvement of skin 2 or more then 2 surface area .
2) stage 3 or 4 lupus nephritis
3) as corticosteroid sparing agent
When female is nonpregnant or not willing to conceive or not breast feeding to baby since MMF is teratogenic.
MMF is indicated in SLE as an effective drug for both remission induction as well as remission maintainance drug..
SIDE EFFECTS:
🚨 GIT : nausea vomiting diarrhea abd pain.
GIT ulceration, CMV associated colitis
🚨 Hematological : pancytopenia .
Pseudo pegler heut anmoly, neutrophils are bilobed rather than hypersegmented.
🚨 Infection : opportunistic , bacterial and viral and fungal
Reactivation of TB
UTI
🚨 Carcinogenic : lymphomas and leukemias
EB viral B cell lymphoma
🚨 Teratogenicity: In early pregnancy : miscarriages
Cranio facial and cardiac malformation in newborn
🚨 Metabolic : high cholesterol , potassium , glucose, blood urea nitrogen
Low calcium and magnesium.
🚨Renal. Frequency, hematuria and sterile pyouria.
Very imp side effect
Contraindications of MMF
Absolute Contraindications:
Pregnancy(causes birth defect = cleft lip and palate,
Microtia with atresia of external auditory canal,micrognathia and hypertelorism anomalies of the distal limbs, heart, oesophagus and kidney)
Hypersensitivity to mycophenolic acid.
Relative contraindications:
Lactation (secreated in breast milk causes potential immunosuppression )
Peptic ulcer disease,
Hepatic
Renal and cardiopulmonary disease.
Commonest prescribed brand name of MMF
Cellcept (by Rouche)
Pack of 50 tablets is available
Price of 1 tablet is 120 to 160 Rs.
Other brand name :
Cycopin
Myfortic
Indications
1) in primary and secondary immunodeficiencies syndromes
2) licensed in UK for the treatment of primary immune thrombocytopenia, GBS and Kawasaki disease
3) off label used either as monotherapy or in combination with other immunomodulating drugs in autoimmune bullous disorders (pemphigus, pemphigoid,EBA, LAD )
Autoimmune connective tissue disease ( dermatomyositis,SS,
SLE)
4) chronic autoimmune urticaria
GVHD and scleromyxoedema
source rooks vol 1 19.35
Infusion related adverse effects:
1.Headache,Fever,fatigue,malaise,shivering ,flushing,dyspnoea
- dermatologic: urticaria, eczema, pompholyx, lichenoid dermatitis
- Arrythmia and hypotension: SVT, bradycardia, tachycardia
- Thrombotic events: stroke, MI, pulmonary embolism
- IgA mediated anaphylaxis and hypersensitivity reactions
- Aseptic meningitis
- Opportunistic infections
- hemolysis
- renal impairment
- transfusion related acute lung injury(TRALI)
RISK FACTORS FOR ACUTE KIDNEY INJURY
- Age>65yrs
- Diabetes mellitus
- Pre existing renal disease
- Dehydration
- RA factor positive
- Cryoglobulin +ve
- use of other nephrotoxic drugs
source: notes and journal
contraindication/Cautions
Contraindication
Sever anaphylaxis resulting from prevoius infusion is contraindication to further use
Presence if risk factor for AKI and thrombosis are relative contraindications
Cautious
IVIG administration may impair efficacy of live atteunated virus vaccines for upto 3 months
Loop diuretics should be avoided before and aftee IVIG infusions
➡️ Dose Regimen
It comes as 5% (50mg/DL) or 10% ( 100mg/DL) liquid or lyophilised preparation.
➡️2g/kg/ cycle , in divided doses over 2-5 days. (means 400mg/kg in 5 days and it can be altered as per severity and indication of disease , like 1000mg/kg /day for 2 days ).
➡️Infusions are started at a rate of 0.5 to 1 mL/kg/hour for the first 15 to 30 minutes, and if no adverse reaction occurs, then the rate can be increased subsequently every 15 to 30 minutes to a maximum of 3 to 6 mL/kg/hour.[
➡️Cycles are repeated at monthly intervals until effective disease control is obtained. (Monthly as half life of IVIG is similar to IgG antibody, which is 28-36 days)
➡️After obtaining disease control, intervals between cycles are gradually increased empirically up to 16 weeks ( 4months).
➡️If remission continues, IVIG can be discontinued.
➡️Premedication :
1- fluid I take should be encouraged during and after the infusion.
2- For general infusion related symptoms, like fatigue, malaise, fever , arthralgia, flushing, rashes,
✅ paracetamol, with or without coedene
✅NSAID
✅Anti-histamine
3- in patients with susceptibility to thrombosis( old age, atherosclerosis, HTN , hypercholesterolemia) :
✅Aspirin
✅LMWH
➡️ PRE-TREATMENT SCREENING
🔆When assessing the risk–benefit ratio of a course of IVIg, factors predisposing the individual to acute kidney injury and thrombosis should be assessed
🔆 Measure serum IgA level
🔆 Thrombophilia screen.
🔆 Before each cycle of IVIg, a full blood count, creatinine level and liver function tests should be checked.
🔆 The hydration of the patient should be optimized, especially for the older person.
🔆 Following medical conditions warrant discussion with your doctor:
Previous blood clots
Dehydration
Diabetes
Kidney disease
Heart disease
Stroke
Pregnant or contemplating pregnancy
Breastfeeding.
➡️ MONITORING
🔆 During and immediately after IVIg infusions, the vital signs, hydration status (to exclude both fluid overload and dehydration) and urine output should be monitored.
🔆 Post‐transfusion, a full blood count with film, and bilirubin level will screen for the possibility of haemolysis.
🔆 Mobility should be encouraged in all patients to minimize the risk of thrombosis.
➡️ Single test to avoid life threatening reaction
🔆 Individuals who have IgA deficiency – patients should be screened before IVIG therapy is instituted, as presence of trace amounts of IgA in IVIg preparations may induce allergic reactions and anaphylaxis in otherwise symptomless IgA‐deficient recipients with circulating anti‐IgA antibodies of IgG or IgE class.
Source: ROOKS chap#19
❇️ trade name is pentaglobin and price of one injection (10ml ) is about 30000/ PKR.
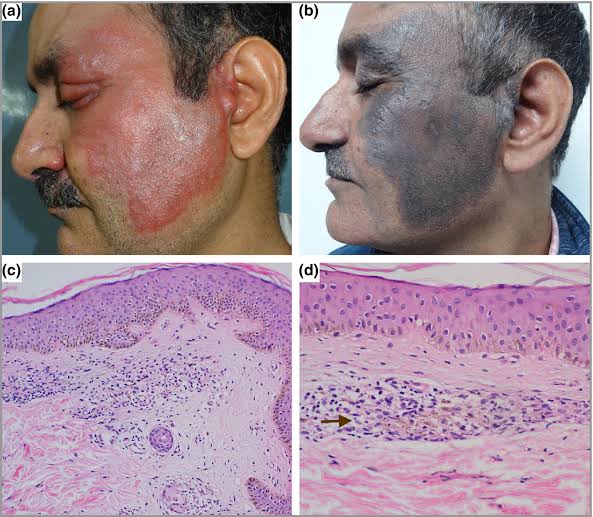
Imp side effect missed is appendicitis like abdominal pain due to deposition of clofazimine in mesenteric lymphnodes





.Mechanism of action of Rituximab and dermatological indications
Rituximab is an IgG1,chimaeric mouse/human anti‐CD20 monoclonal antibody .
Following binding of the rituximab Fab fragment to CD20, B‐cell lysis occurs, primarily via antibody‐ dependent cellular cytotoxicity mediated by one or more of the Fcγ receptors on the surface of granulocytes, macrophages and NKγ cells, although other mechanisms have also been implicated.
Naïve B cells disappear rapidly from peripheral blood circulation and also,
to a more variable extent, from tissues, depending on the pathology and setting.
Contraindications
- Known hypersensitivity to rituximab
- Active severe infection
- HIV with CD4 <250 cells/mm3
- Severe heart failure
- History of bronchospasm, hypotension, and
angioedema
- Pregnancy (FDA category C)
- Lactation
Warning
Women with childbearing potential should use effective contraception during treatment and continue the same for at least 12 months after the last dose.
Treatment response
Biweekly during the 1st month, once a month till 6 months after the 4th dose or complete withdrawal of steroids, whichever is later, once in 3 months for the next 1 year followed by once in 6 months for 1 year and once a year thereafter
* Complete hemogram every 3 months
* Repeat serology for antidesmoglein 1 and 3 antibodies at
month 6, 12, and 24 and when there is clinical evidence of relapse
🟢 Pretreatment labs
🔵Screening prior to rituximab should aim to identify in particular those with cardiac disease and any current or past infection at risk of progression or reactivation. This includes a past history of tuberculosis, risk factors for or presence of active infection together with relevant screening tests for latent tuberculosis and blood‐borne viral infections (HIV, hepatitis B and C). Fulminant hepatic failure following hepatitis B reactivation is well documented in the oncology literature, although rituximab may not be absolutely contraindicated in the presence of positive hepatitis B or C serology, given appropriate antiviral prophylaxis (for hepatitis B) and relevant expert hepatology advice.
Vaccination status against Pneumococcus and influenza as well as any travel plans likely to require live vaccination should be checked.
The importance of avoidance of pregnancy during and for 12 months post‐infusion should be emphasized.
Commonly recommended additional investigations include full blood count, renal and liver function, serum immunoglobulins and lymphocyte subsets.
🟢 Monitoring during infusion
🔵1.Check pulse, BP and respiratory rate and do pulse oximetry every 30 minutes
- Watch for infusion reactions ( fever, rigors, nausea, vomiting, headache, hypotension and abdominal pain)
- On most occasions, infusion reactions can be managed by reducing the speed of infusion ( half the flow-rate and re-administer the premedication)
- Serious adverse effects such as angioedema and anaphylaxis require complete cessation of infusion.
- Adequate hydration should be ensured immediately after infusion.
Monitoring for rituximab
-Infections
-Neurological disturbances
-Routine baselines ..monthly
-Immunoglobulin / lymphocyte subset prior to any subsequent infusion
Cardiological assessment
Labs for monitoring :
CBC
LFTs, Rfts
IGRA
CXR
Viral markers:HBV, HCV, HIV
ECG
Contraindications
Some absolute and relative contraindications of the drug include
1)Severe, active infection
2)Hypersensitivity to any of the components of the formulation
3)Severe heart failure
4)Uncontrolled cardiac disease
5)Pregnancy
DERMATOLOGICAL USES:
FDA approved
- Pemphigus Vulgaris
- Granulomatosis with polyangiitis
- Microscopic polyangiitis
- Rheumatoid Arthritis
Others
- Cutaneous B-cell lymphoma
- Primary blistering diseases
- Dermatomyositis
- Graft versus host disease
- Cutaneous vasculitis
- Atopic dermatitis
- Lupus erythematosus
- Eosinophilic fasciitis
MECHANISM OF ACTION
◇ Rituximab is an immunoglobulin G1 (IgG1) kappa monoclonal antibody composed of a murine (mouse) variable region (Fab portion) that is fused onto a human constant region (Fc portion)
◇Fab portion binds to CD20 antigen on surface of pre-B and mature B lymphocytes
◇Fc portion then recruits immune cells that destroy these lymphocytes
Mechanisms may include:
- Complement-dependent cytotoxicity
- Antibody-dependent cell-mediated cytotoxicity
- Apoptosis
imunocompromised patients and ask pts to avoid live vaccination within 4 weeks of infusion.
Avoid pregnancy 12mnths post rituximab infusion.
Brand and prices
🌻 In Pemphigus we follow Lymphoma protocol(shared below)
🌻Ristova Injection 500mg
Price:85,500
🌻Mabthera Injection 500mg
Price: 114,000
🌻https://medicalstore.com.pk/formula/rituximab/

,Recommeded duration of Contraception during different immunosupressives & biologics in Pregnancy…..
💊 TNF@inhibotors should be used only 1st 12 weeks of pregnancy
💊 Certolizumab can be used throughout pregnancy
💊 Consider using ciclosporin or certolizumab pegol as first-line options when it is necessary to start a systemic therapy during the second or third trimester.
💊 Acitretin> 3 years contraception needed after cessation of therapy
💊 Isotretinoin> 1 month contraception for females after cessation of therapy; no contraception needed for male patients
💊 MTX > 3 months of contraception needed after cessation of therapy for both male n female (its 6months in rooks)?
💊 Cyclophosphamide> 03 – 06 months of contraception needed after cessation of therapy
Click Here to View the BIOLOGICALS IN DERMATOLOGY
Click Here to View the GUIDELINES OF CARE WITH BIOLOGICS
Mechanism of action.
The systemic antifungal drugs can be broadly classified by their mechanistic properties into those that
👉🏻Act on the fungal wall or cell membrane
👉🏻Act intracellularly.
🟩Fungal wall/cell membrane agents are subdivided:
👉🏻Those that inhibit ergosterol function
👉🏻Those that inhibitβ‐glucan synthase.
👉🏻ERGOSTEROL INHIBITORS:
are categorized :
- azoles (inhibitors of lanosterol 14‐α demethylase, essential for the synthesis of ergosterol)
B.allylamines (inhibitors of squalene epoxidase, also essential in ergosterol synthesis, such as terbinafi ne )
C.polyene antifungals (such as nystatin and amphotericin , which bind to ergosterol and interfere with the integrity of the fungal cell membrane).
🔅 Azoles are further subdivided into:
A.Imidazoles
B.triazoles ( including
fluconazole,itraconazole , posaconazole and voriconazole ).
🟩β‐glucan synthase inhibitors interfere with the synthesis of glucan present in cell wall. (anidulafungin , caspofungin and micafungin ).
🟩Antifungal drugs with intracellular mechanisms of action
🔅Flucytosine: a pyrimidine analogue that inhibits fungal DNA and RNA synthesis
🔅Griseofulvin
inhibits fungal mitosis by binding to tubulin and thereby disrupting microtubule function.
Side effects
Hepatotoxicity
GI side effects
Bone marrow suppression
Prolongs QT interval mainly voriconazole
Contraindications
Known allergy to any antifungal
Pregnancy and lagtation
Liver or kidney disease
Investigation before starting
CBC
Lft
Pregnancy test
And repeat them after 4-6weeks of treatment.